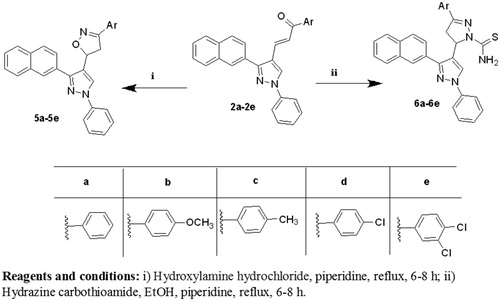
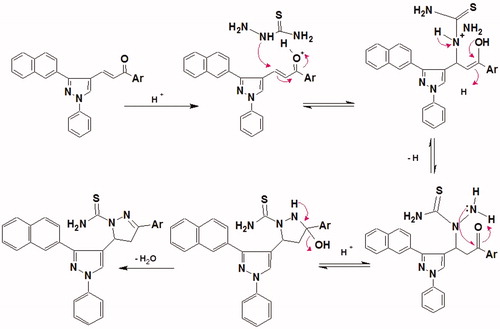
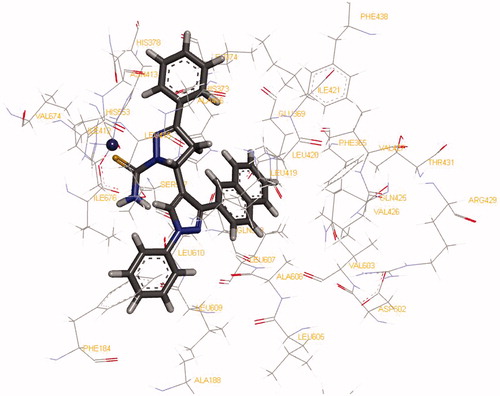
Figures & data
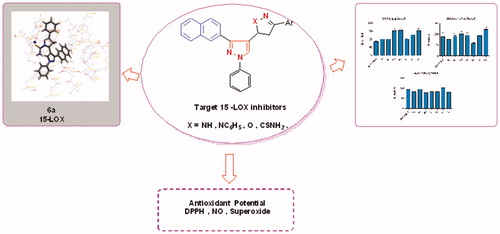
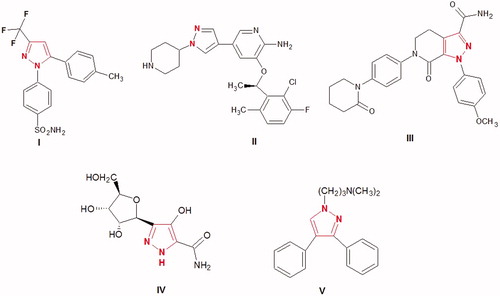
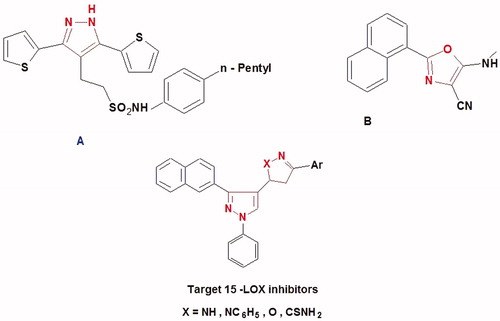
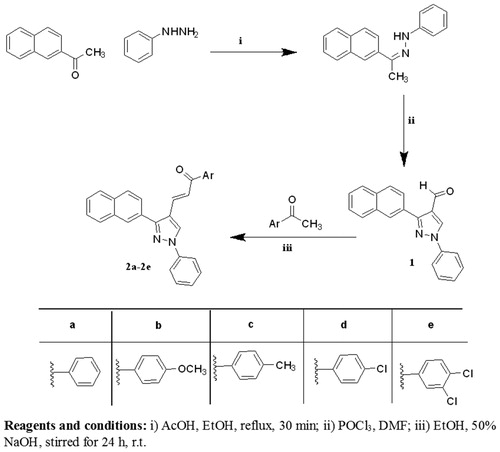
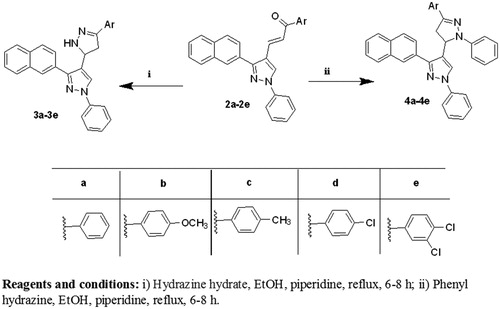
Figure 2. Structure of the lead antioxidant pyrazole derivatives and the designed target compounds 2–6.
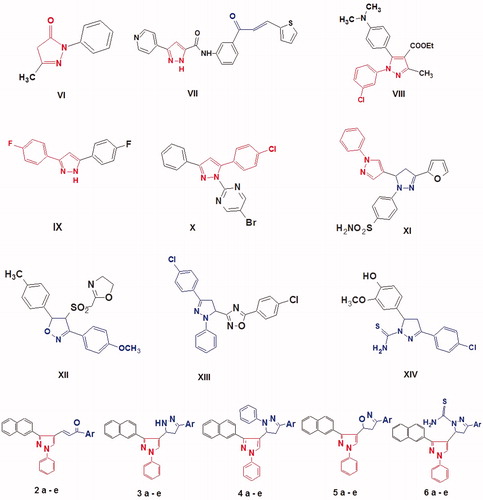
Table 1. In vitro antioxidant potential and 15-LOX inhibition activity of compounds (2–6).
Figure 5. Structure activity relationship of the pyrazole derivatives against DPPH radical scavenging assay.
Figure 6. Effect of compounds (3a, 4e, 5 b, 5c, 6a, 6c, 6e) and ascorbic acid on the endogenous antioxidant status of rats. GSH: reduced glutathione; TBARS: thiobarbituric acid reactive substances. Data are expressed as mean ± SEM% control. (n = 6). *, **, and *** p < 0.05, p < 0.01, and p < 0.001 compared to control group.
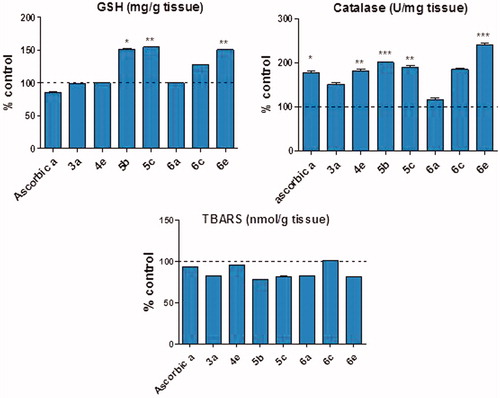
Table 2. In vivo antioxidant potential of compounds.