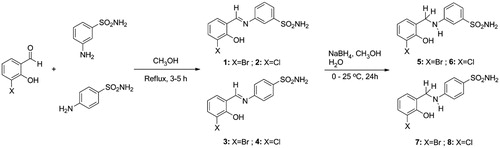
Figures & data
Table 1. KI values of hCA I, II and AChE with derivatives 1–8, AAZ and TAC as standard inhibitors.
Table 2. Selectivity index values for KI values of the compounds 1–8.
Table 3. The radical scavenging and metal reduction activity of synthesised compounds (1–8).
Table 4. ADMET–related parameters of the derivatives (1–8).
Figure 1. Interaction of the ligands with the key amino acids within the active site of hCA I (PDB ID: 4WUP). (A) Docking pose of the native ligand 3UF (4-[(2-hydroxyethyl)sulfanyl]benzenesulfonamide, PubChem CID: 4269754). (B) Docking pose of compound 8 (4-((3-chloro-2-hydroxybenzyl)amino)benzenesulfonamide).
![Figure 1. Interaction of the ligands with the key amino acids within the active site of hCA I (PDB ID: 4WUP). (A) Docking pose of the native ligand 3UF (4-[(2-hydroxyethyl)sulfanyl]benzenesulfonamide, PubChem CID: 4269754). (B) Docking pose of compound 8 (4-((3-chloro-2-hydroxybenzyl)amino)benzenesulfonamide).](/cms/asset/8a130b3b-e8c1-4d02-af0c-385981e1a9da/ienz_a_1746784_f0001_c.jpg)
Figure 2. Interaction of the ligands with the key amino acids within the active site of hCA II (PDB ID: 4HT0). (A) Docking pose of the native ligand V50 (4-[(4,6-dimethylpyrimidin-2-yl)thio]-2,3,5,6-tetrafluorobenzenesulfonamide, PubChem CID: 71299336). (B) Docking pose of compound 7 (4-((3-bromo-2-hydroxybenzyl)amino)benzenesulfonamide).
![Figure 2. Interaction of the ligands with the key amino acids within the active site of hCA II (PDB ID: 4HT0). (A) Docking pose of the native ligand V50 (4-[(4,6-dimethylpyrimidin-2-yl)thio]-2,3,5,6-tetrafluorobenzenesulfonamide, PubChem CID: 71299336). (B) Docking pose of compound 7 (4-((3-bromo-2-hydroxybenzyl)amino)benzenesulfonamide).](/cms/asset/92bac284-2ecc-44dd-8820-208e413c6224/ienz_a_1746784_f0002_c.jpg)
Figure 3. Interaction of the ligands with the key amino acids within the active site of AChE (PDB ID: 4EY7). (A) Docking pose of the native ligand E20 (1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine, PubChem CID: 1150567). (B) Docking pose of compound 2 (3-((3-chloro-2-hydroxybenzylidene)amino)benzenesulfonamide).
![Figure 3. Interaction of the ligands with the key amino acids within the active site of AChE (PDB ID: 4EY7). (A) Docking pose of the native ligand E20 (1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine, PubChem CID: 1150567). (B) Docking pose of compound 2 (3-((3-chloro-2-hydroxybenzylidene)amino)benzenesulfonamide).](/cms/asset/046b0168-cc4a-49c8-88af-588793356734/ienz_a_1746784_f0003_c.jpg)