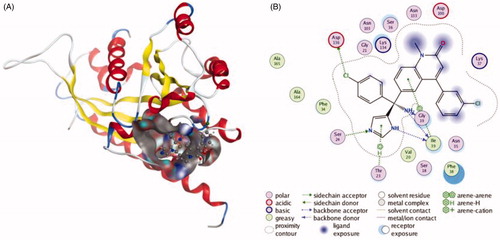
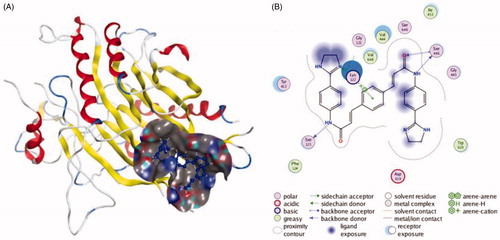
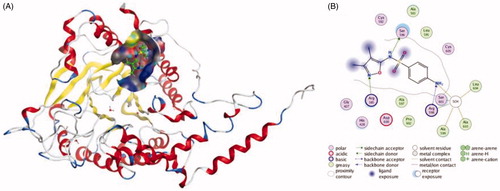
Figures & data
Table 1. RAB27A inhibitors.
Table 2. nSMase inhibitors.
Table 3. Other inhibitors.