Figures & data
Figure 1. Molecular docking of the active compounds 9, 10 and 5FU against Thimidylate synthase (TS) protein 6QXG. A: Binding mode of 9, 10 and 5FU at TS binding site 3D plot. B: Binding mode of 9, 10 and 5FU at TS binding site 2D plot. 5FU: -5-fluorouracil.
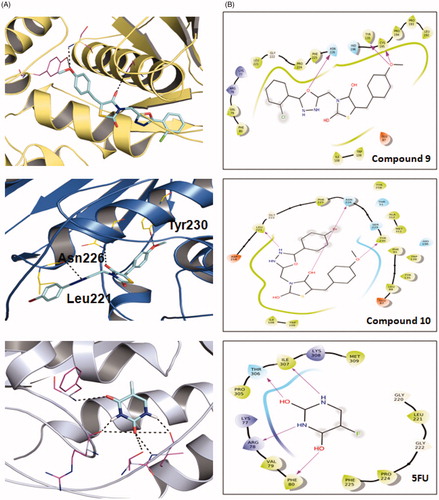
Table 1. Pharmacokinetics/ADME predictions of the target compounds 7–21.
Table 2. The IC50 (µM) of the synthesised compounds (7–21) against tested human cancer cell line (MCF-7 and HCT-116)Table Footnotea.
Table 3. In vitro thymidylate synthase (TS) activity of the active compounds 9, 10, 14, 15 and PTX.
Table 4. Docking scores of active compounds 9 and 10 against human thymidylate synthase protein 6QXG.