Figures & data
Figure 1. The simplest coumarin, compound 1, its hydrolysis product 2, and the natural product coumarin 3 (and its hydrolysis product 4) for which the CA inhibitory activity was first reportedCitation6.
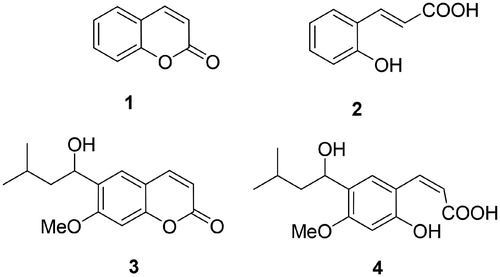
Figure 2. CA inhibition mechanisms (A–D) and the CA activation mechanism (E). The CA modulators of activity incorporate various scaffolds and tails in their molecule, as well as characteristic functionalities for all category: (A) the zinc binders possess a zinc-binding group (ZBG) which is coordinated to the metal ion; (B) the compounds which anchor to the zinc-coordinated water incorporate an anchoring group (AG) which hydrogen bonds with the water coordinated to the metal and the OH of Thr199; (C) AGs are also present in compounds which occlude the entrance of the active site cavity; (D) some inhibitors which bind out of the active site; (E) the activators incorporate proton-shuttling moieties (PSMs) and bind in the same active site region as the inhibitors shown at (C). Only α-class CAs are considered here (as they are the enzymes present in mammals, including humansCitation12,Citation14) although these mechanisms may be valid to other CA classes.
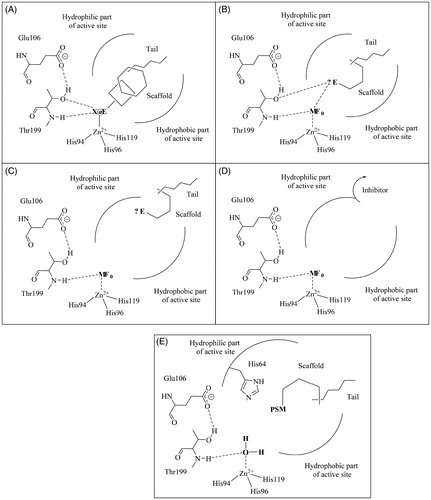
Figure 3. X-ray crystal structure of adducts of coumarins 1Citation10 and 3Citation6 bound to hCA II. The hydrolysis products of the two coumarins, cis-2-hydroxycinnamic acid 2 (in yellow) and trans-2-hydroxy-cinnamic acid 4 (in magenta) were observed bound wat the entrance of the CA active site. The catalytic Zn(II) ion is the central violet sphere, its three protein ligands (His94, 96 and 119, CPK colors) and the proton shuttle residue His64 (in red) are evidenced. The hCA II backbone is shown as the green ribbon, with its various α-helices, β-sheets, and loops represented in a canonical manner.
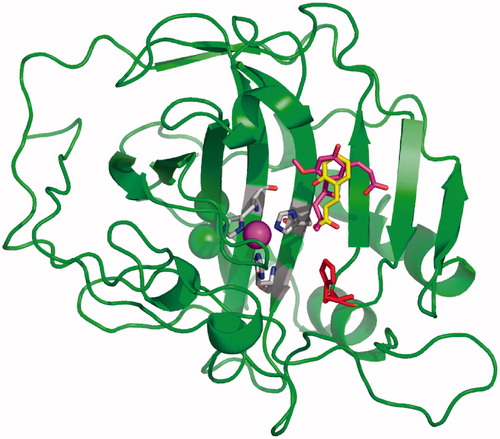
Table 1. Inhibition data against six CA isoforms (hCA I, II, VII, IX, XII, and XIII) with coumarins 5–35Citation9.
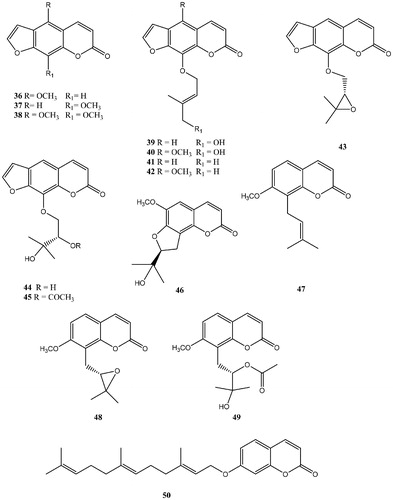
Table 2. hCA I, II, IX, and XII inhibition with NPCs 36–50.
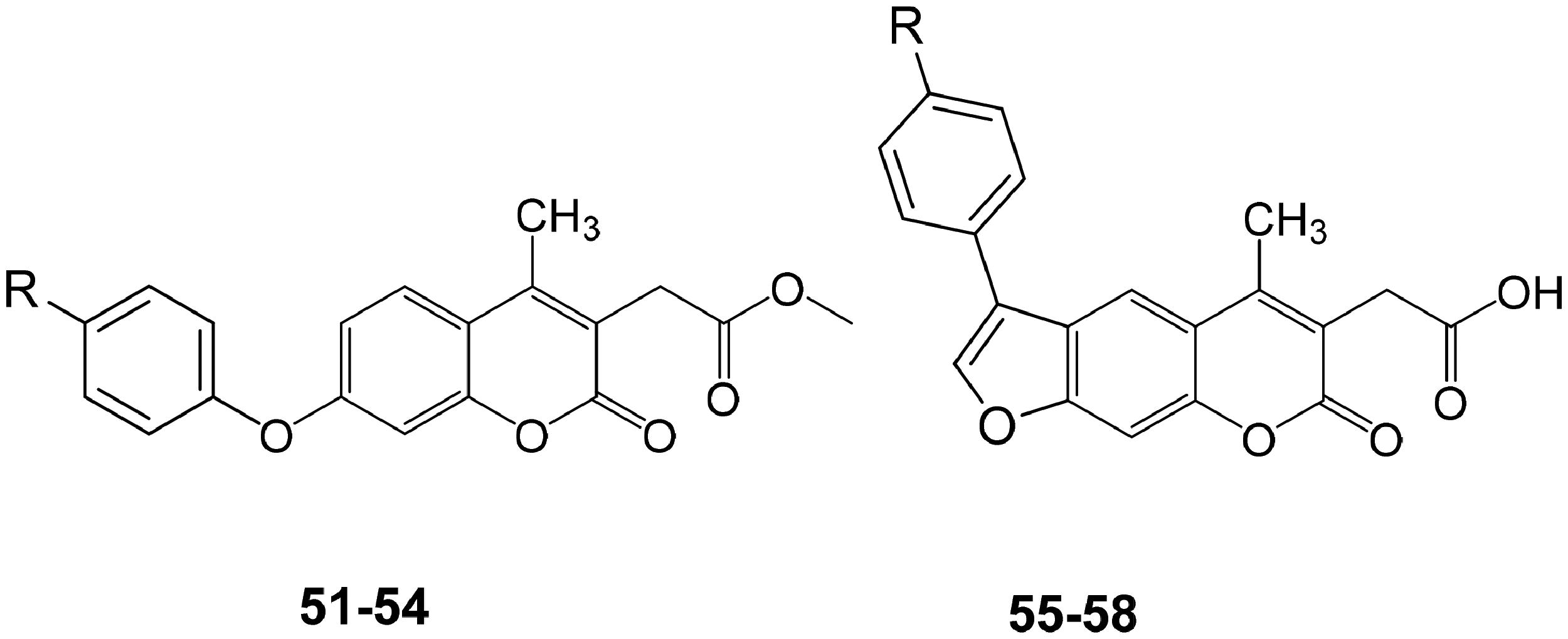
Table 3. Coumarins and psoralens 51–58 and their hCA I, II, IX, and XII inhibitory action.