Figures & data
Figure 1. Sulphatide synthesis by CST: galactosylceramide (cerebroside) is converted to sulphatide by CST in the presence of PAPS as sulphate donor.
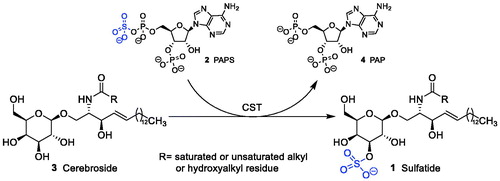
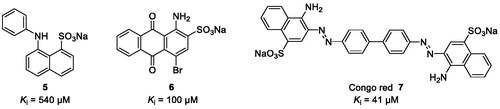
Figure 2. Chemical structures of aromatic dyes known as CST inhibitors competing with the co-substrate PAPS.
Scheme 1. Reagents and conditions: (a) (i) PMe3, THF, then H2O; (ii) appropriate RCOOH, EDC, CH2Cl2; (b) H2, Pd black; (c) (i) PMe3, THF, then H2O; (ii) (Boc)2O, Et3N, CH2Cl2; (d) (i) HCl, AcOH, H2O; (ii) nervonic acid, oxalyl chloride, reflux; the acyl chloride is added to crude amine in THF/aq. NaOAc.
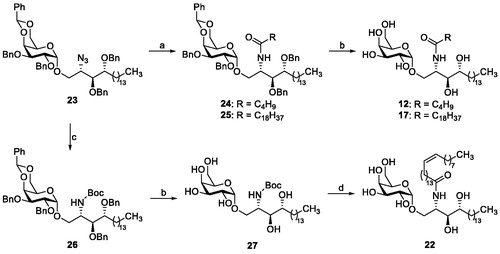
Table 1. Investigation of analogues of galactocerebroside as substrates of CSTa.
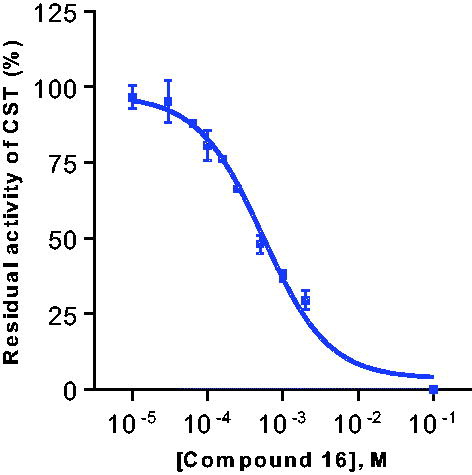
Figure 3. Enzyme kinetics of galactosylceramide sulphotransferase for selected substrates. For Km and Vmax values see .
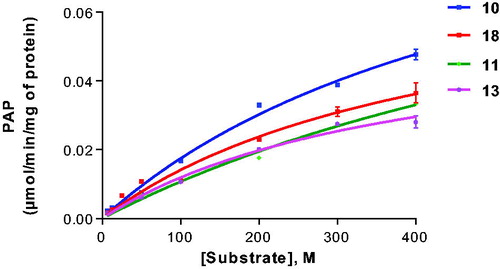
Table 2. CST inhibitory activity of galactocerebroside analogues