Figures & data
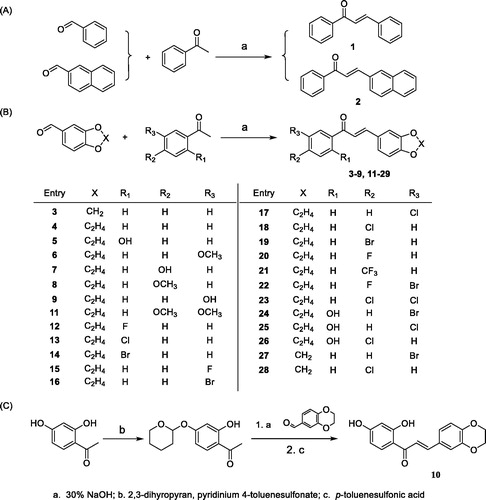
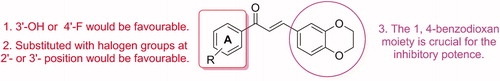
Figure 1. (A) Structures of irreversible and reversible MAO inhibitors in clinical use; (B) Structures, potencies and inhibition modes of previously described MAO-B inhibitors. Middle, design strategy of 1, 4-benzodioxan-substituted chalcone compounds.
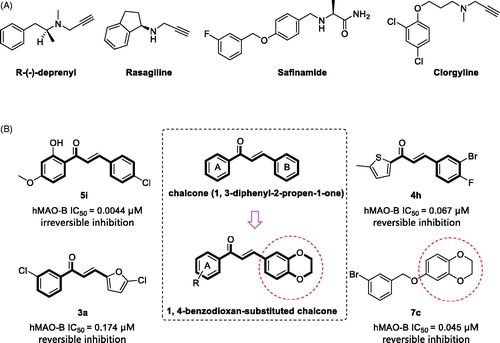
Table 1. MAO inhibitory activities of 1, 4-benzodioxan-substituted chalcone derivatives
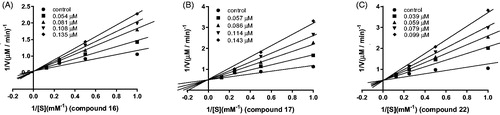
Figure 4. Time-dependent inhibition of hMAO-B by reference compounds R-(–)-deprenyl, rasagiline and safinamide (0.05 μM, 0. 20 μM and 0.06 μM, respectively, A) and test compounds 16, 17, and 22 (0.28 μM, 0.28 μM and 0.19 μM, respectively, B). The remaining activity was expressed as % of activity.
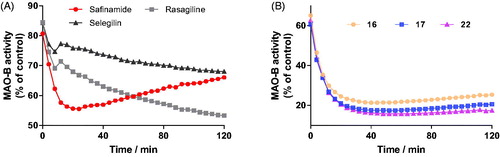
Figure 5. Docking poses of chalcone compounds 1, 4, 16, and 22 in the hMAO-B (PDB code: 2V61) (A–C) or hMAO-A (2Z5X) (D) active site. (A) docking poses of compounds 1 (gray) and 4 (yellow); (B) docking poses of compounds 4 (yellow), 16 (cyan), and 22 (brown); (C) the docking pose of 22 in the active site of hMAO-B; (D) the docking pose of 22 in the active site of hMAO-A. Hydrogen bonds are shown as yellow dotted lines, halogen bonds are shown as blue dotted lines. For clarity, only the relevant residue side chains are shown. FAD is rendered as white sticks. Red bows indicate steric hindrance.
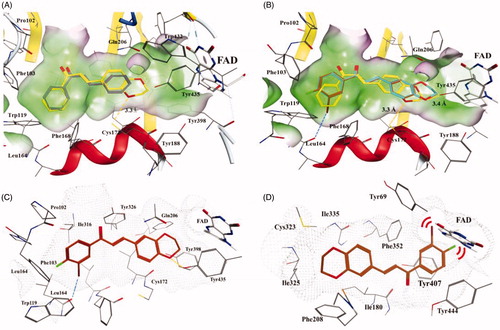
Table 2. Calculation of the drug-like properties of selected active compounds and MAO-B standard inhibitors
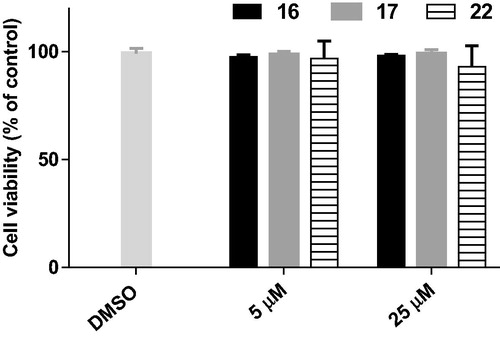
Figure 6. Cell viability of BV2 cells after treatment with compounds 16, 17, and 22 at the concentrations of 5 and 25 µM for 24 h.