Figures & data
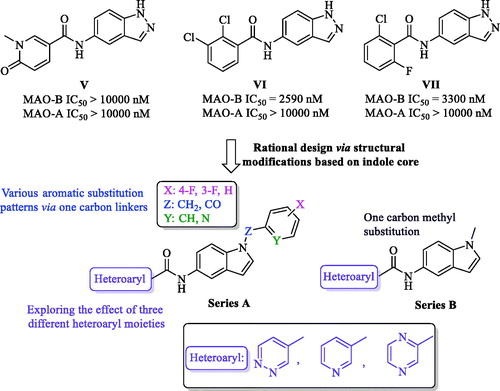
Scheme 1. Reagents and conditions: (a) appropriate halide derivative, NaH (60% in oil), DMF, 0–100 °C, 24 h; (b) dimethyl carbonate, K2CO3, DMF, reflux, 3 h; (c) NH4Cl, Fe, EtOH/H2O, reflux, 1 h; (d) appropriate heteroaryl carboxylic acid, DIPEA, HATU, DMF, MW, 116 °C, 45 min.
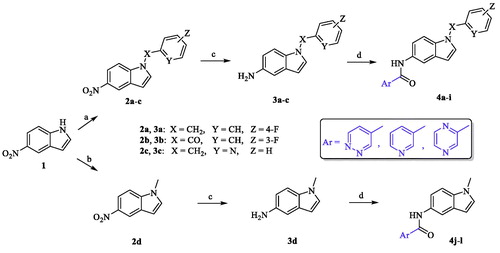
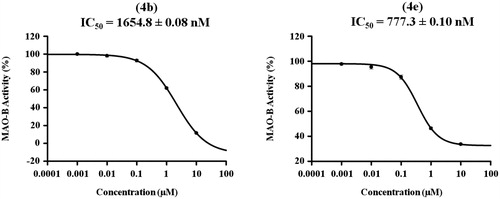
Table 1. Inhibitory effects of the synthesized compounds against MAO-B.
Table 2. Inhibitory effects of the compounds 4b and 4e against MAO-A.
Figure 4. Type of inhibition of MAO-B by compound 4e. The catalytic rates were measured at different concentrations of benzylamine (0.065, 0.125, 0.25, 0.5, 1, 2, and 4 mM) in the absence and in the presence of different concentrations (10, 30, and 100 nM) of compound 4e. The Vmax, Km, and Ki values were calculated using SigmaPlot®.
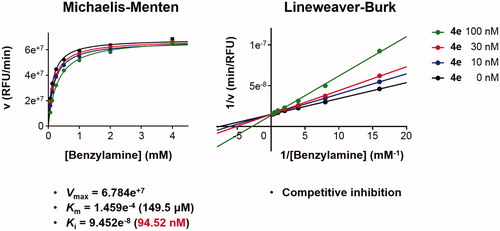
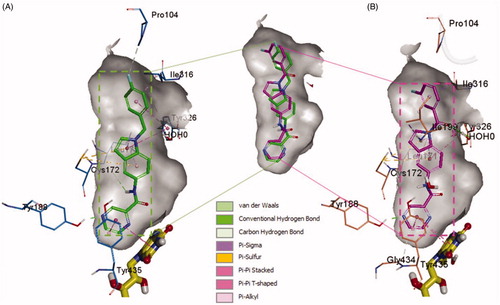
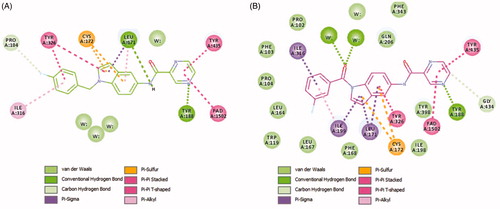
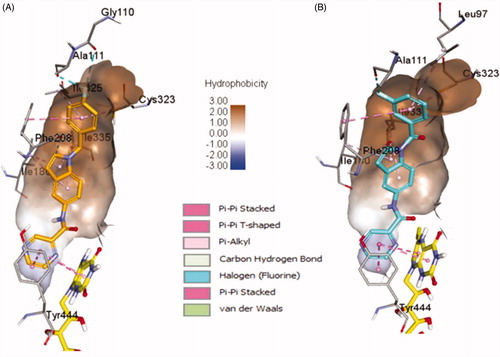
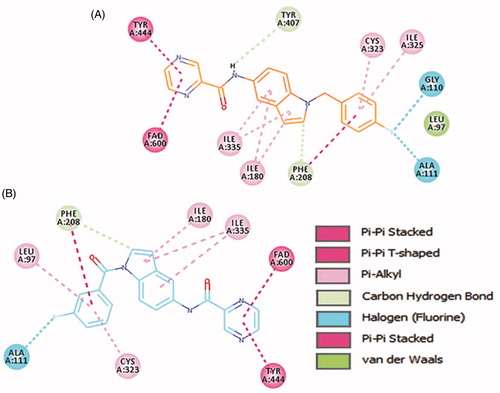
Figure 5. The docked model of the most active compounds 4b (A) and 4e (B) into MAO-B binding pocket.