Figures & data
Figure 1. XRPD diffraction patterns of curcumin, piperine, their physical mixture (ph. m.) and system with hydroxypropyl-β-cyclodextrin (HP-β-CD) obtained by kneading method.
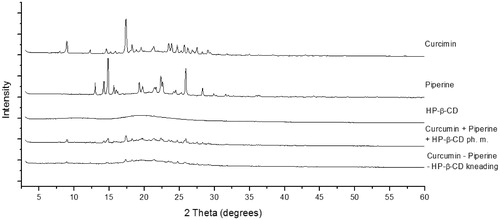
Figure 2. DSC thermograms of curcumin, piperine, their physical mixture, and system with hydroxypropyl-β-cyclodextrin (HP-β-CD) obtained by kneading method.
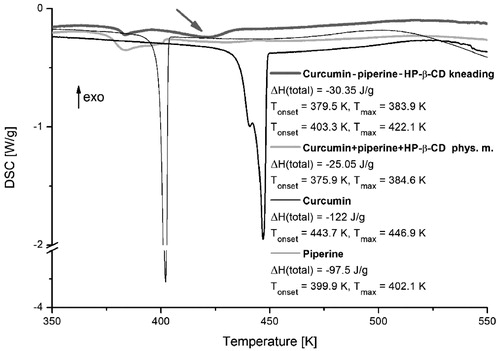
Figure 3. The experimental FT-IR of curcumin, piperine, their physical mixture, and system with hydroxypropyl-β-cyclodextrin (HP-β-CD) obtained by kneading method.
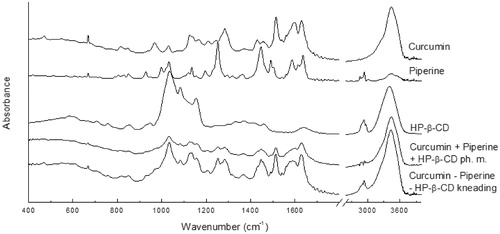
Figure 4. CP MAS 13C NMR spectra of HP-β-CD (blue), curcumin (green), piperine (red), kneaded compounds (black), and physically mixed compounds (magenta).
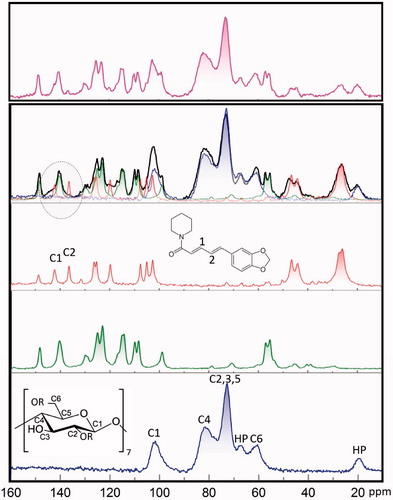
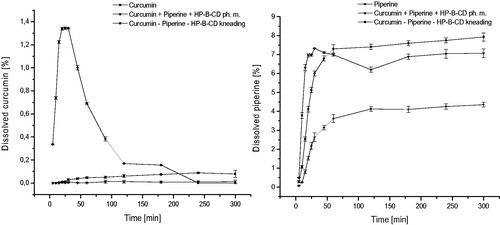
Figure 6. Values of apparent permeability coefficients of curcumin determined for gastrointestinal permeability (A) and permeability through the blood–brain barrier (B).
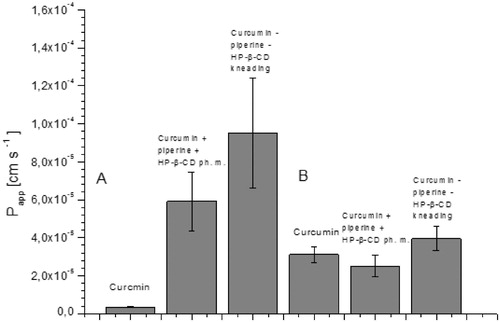
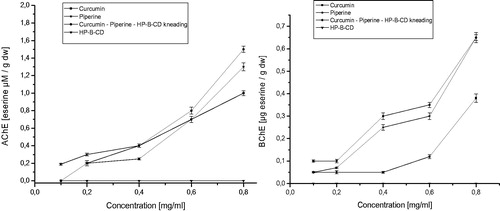
Figure 7. Values of apparent permeability coefficients of piperine determined for gastrointestinal permeability (A) and permeability through barrier blood–brain (B).
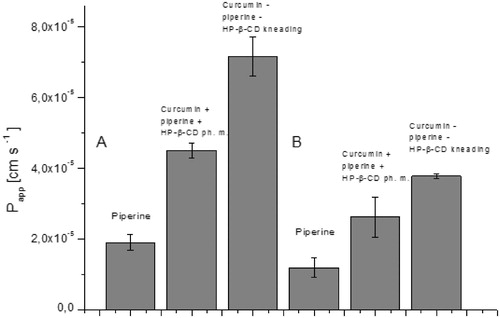
Table 1. Antimicrobial activity of curcumin, piperine and curcumin – piperine – 2-hydroxypropyl-β-cyclodextrin system in the concentration 25 mg/mL when dissolved in methanol and filtered.