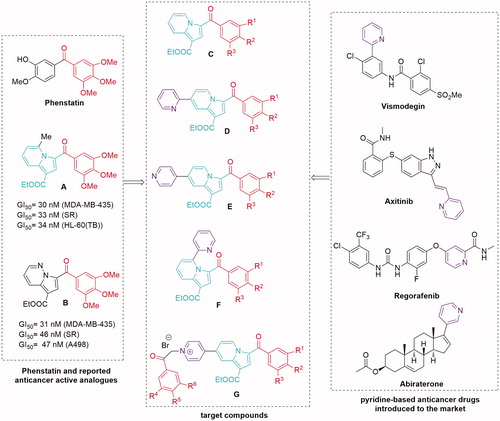
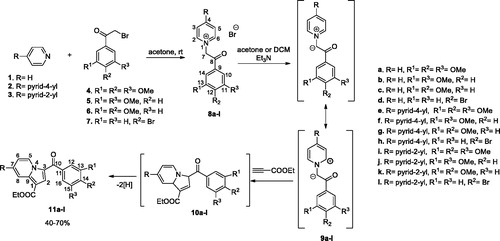
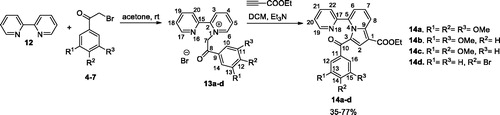
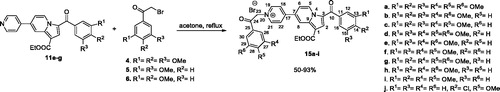
Figures & data
Figure 2. Effects of compounds 11a, 11b, 11k, 14a, 15a, and 15j (10−5 M) on microtubule dynamics using Paclitaxel (10−5 M) as microtubule stabilising agent and Phenstatin (10−5 M) as microtubule destabilising agent.
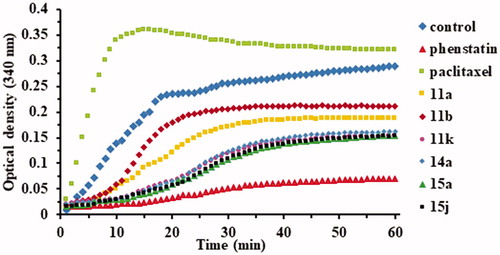
Table 1. Results of the in vitro growth inhibition (GI %) of tested compounds against human cancer cell lines in the single-dose assayTable Footnotea.
Table 2. Results of the 5-dose in vitro human cancer cell growth inhibitionTable Footnotea for compounds 11a, 15a, and 15j and positive control Phenstatin.
Table 3. Binding orientation, energy, and amino acid contacts for tested compounds, as predicted by molecular docking experiments.