Figures & data
Figure 1. The two moieties combined to synthesise the novel MTDLs in this study. (a) Edaravone. (b) R-substituted N-benzyl pyridinium.
Figure 2. Edaravone-N-benzyl pyridinium hybrid compounds designed and evaluated in this study. R = H, Br, F, Cl, or CH3.
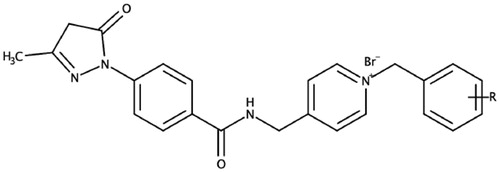
Figure 3. The active site cavity of AChE exhibiting the binding and interactions of representative compound 5f (Scheme 1). (a) Two-dimensional (2D) representation of the docked compound 5f. The close proximity of the Arg 289 residue to edaravone’s pyrazoline ring can be observed. (b) Three-dimensional (3D) representation of the docked compound 5f, showing the orientation and positing of 5f within the AChE active site cavity.
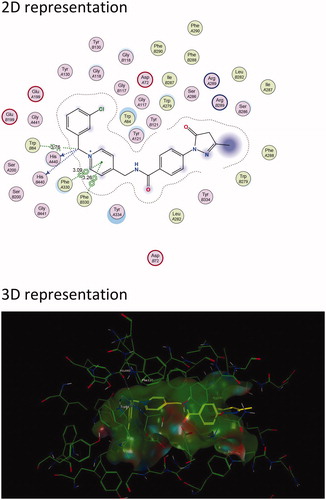
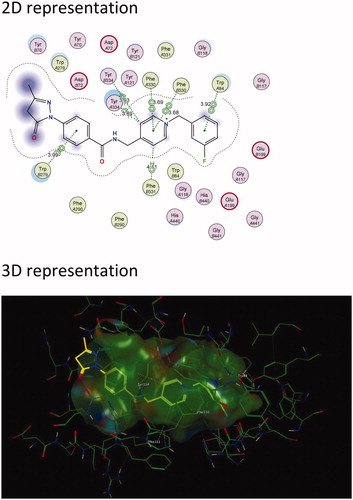
Figure 4. The active site cavity of AChE exhibiting the binding and interactions of representative compound 5c (Scheme 1). (a) 2 D representation of the docked compound 5c. Interactions with Trp 279 (PAS) and Trp 84 (CAS) can be observed. (b) 3D representation of the docked compound 5c, showing the orientation and positing of 5c within the AChE active site cavity.
Scheme 1. Synthesis pathway of the edaravone-N-benzyl pyridinium derivatives 5a–5l. Reagents and conditions: (i) HATU, DMF, DIPEA, 2 h, stirring under reflux. (ii) DMF, stirring under reflux, 4–6 h at 40–50 °C. (1) Edaravone-COOH, (2) 4-(aminomethyl) pyridine, (4) R-benzyl bromide derivatives.
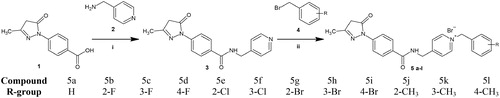
Figure 5. Two major tautomeric forms of the edaravone-N-benzyl pyridinium hybrid compounds and their respective 1H NMR chemical tautomeric shifts in deuterated solvents.
Figure 6. Overlaid 1H NMR spectra of compound 5g in methanol-d4 (blue) and DMSO-d6 (red). (a) Singlet that represents the CH-group of the amine tautomer.
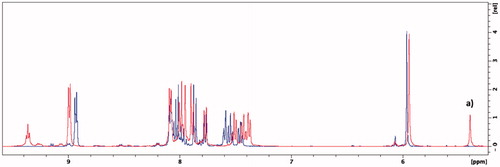
Table 1. In silico BBB predictions and IC50 values (µM) of the test compounds and controls for eeAChE, eqBuChE, and DPPH+.
