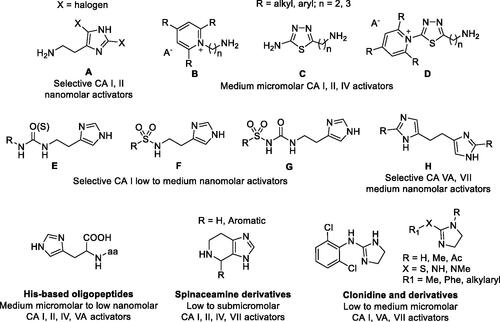
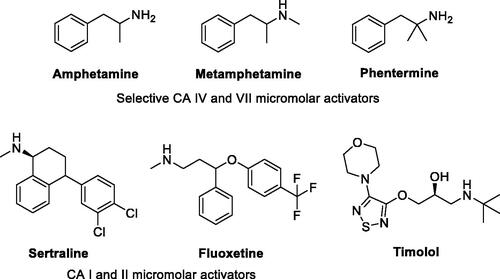
Figures & data
Figure 1. Natural amino acids and amines investigated for the activation of catalytically active hCA isoforms.
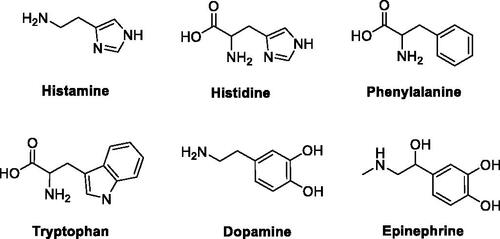
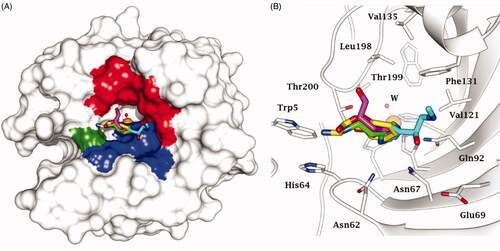
Figure 2. Superimposition of CAA – hCA II complexes as determined by X-ray crystallography. (A) Surface view: the hydrophobic half of the active site is coloured red; HisCitation64 is coloured green and the hydrophilic half in blue. (B) Ribbon active site view. The activators are histamine, in green (PDB 1AVN); L-His, in magenta (PDB 2ABE); L-Phe, in gold (PDB 2FMG); adrenaline, in cyan (PDB 2HKK).
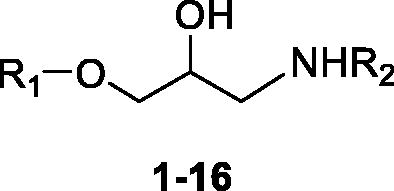
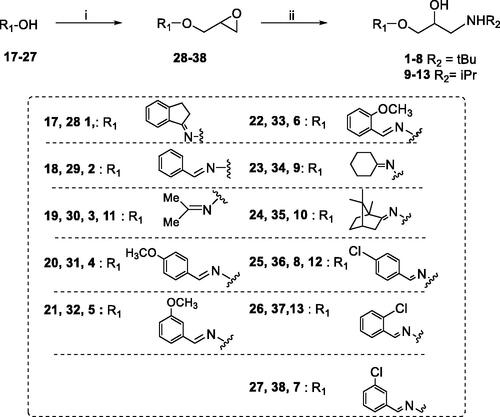
Scheme 1. Preparation of Amino alcohols 1–13. Reagents and conditions: (i) epichlorohydrin, MeONa/MeOH, dry DMF, 60 °C, 1 h; (ii) iPrNH2 or tBuNH2, Benzene, 90 °C, 12 h.
Scheme 2. Preparation of Amino alcohols 14–16. Reagents and conditions: (i) epichlorohydrin, Et3N, dry DMF, rt, 18 h; (ii) iPrNH2 or tBuNH2, Benzene, 50 °C, 4 h; (iii) NH3 MeOH 7 N, rt, 2 h; (iv) o-, m-, p-hydroxybenzaldehyde, EtOH, 90 °C, 12 h.
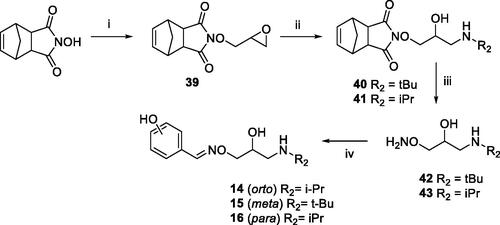
Table 1. Activation data of human CA isoforms I, II, IV and VII with amino alcohols 1–16 and histamine as reference CAA by a stopped flow CO2 hydrase assayCitation34.