Figures & data
Figure 1. (A) TvaCA1 dimeric structure, with the two monomers shown in green and red, respectivelyCitation10. (B) Active site of the enzyme, with the zinc ion (gray sphere) coordinated by two Cys, one His and one water molecule/hydroxide ion (shown in red). Residues numbering as described by Urbański et al.Citation10.
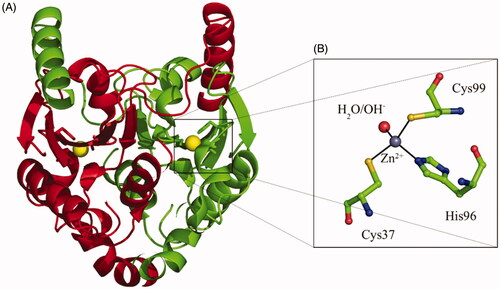
Table 1. Inhibition of human isoform hCA II, for comparison, and of the protozoan enzyme TvaCA1 with sulphonamides 1–24 and the clinically used drugs AAZ–HCT, measured by a CO2 hydrase, stopped-flow assay.Citation21