Figures & data
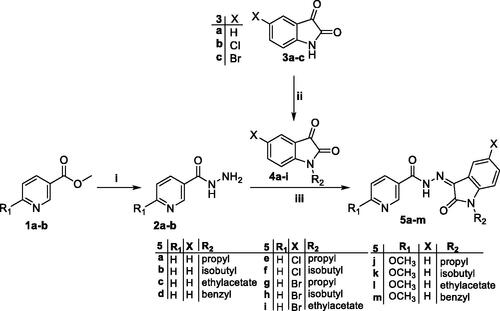
Scheme 1. Synthesis of target isatin hybrids 5a–m; (i) NH2NH2.H2O/methanol/reflux 4 h, (ii) R1-Br/DMF/KI (Cat.)/K2CO3/reflux 3 h, (iii) Ethanol absolute/drops glacial acetic acid (Cat.)/reflux 6 h.
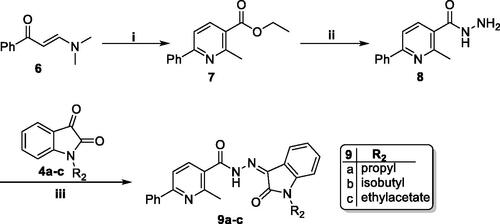
Scheme 2. Synthesis of target isatin hybrids 9a–c; (i) ethyl acetoacetate/NH4OAc/glacial acetic acid/reflux 6 h, (ii) NH2NH2.H2O/methanol/reflux 6 h, (iii) Ethanol absolute/drops glacial acetic acid (Cat.)/reflux 6 h.
Scheme 3. Synthesis of target compound 14; (i) NaOH/MeOH/reflux 6 h, (ii) SOCl2/reflux 4 h, (iii) NaN3/acetone/stirring at R.T. 4 h, (iv) Dry toluene/reflux 1 h, (v) Dry toluene/reflux 5 h.
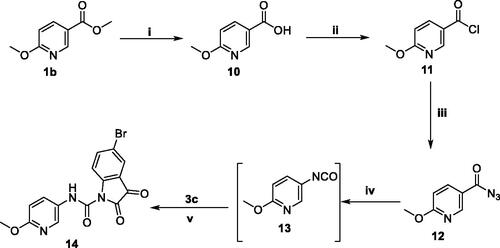
Table 1. MIC (µg/mL) for hybrids (5a–m, 9a–c, and 14) against M. tuberculosis (ATCC 27294) and Isoniazid/Streptomycin resistant M. tuberculosis (ATCC 35823), and LogP measurements for hybrids (5a–m, 9a–c, and 14).
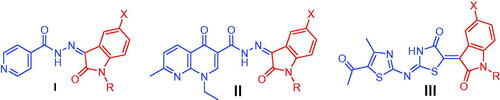
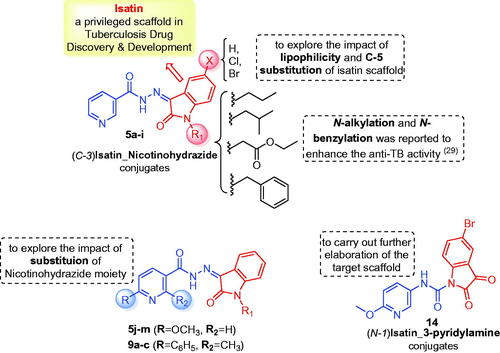
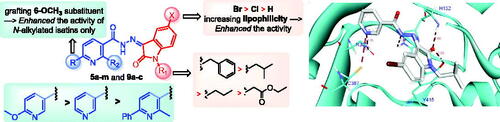
Figure 3. Summary for the structure activity relationships for anti-mycobacterial activity of the target hybrids.
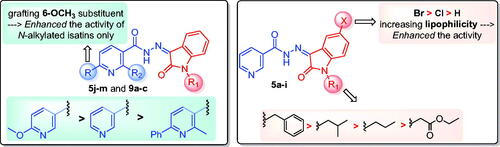
Table 2. MIC (µg/mL) for hybrids (5a–m, 9a–c, and 14) against bronchitis causing-bacteria as determined using XTT assay.
Table 3. In vitro cytotoxic effect for hybrids 5g and 5h towards non-tumorigenic WI-38 cells, and their Selectivity index (S.I.).