Figures & data
Figure 1. Amino acids and amines 1–24 investigated as CAAs against TvaCA1. Some β-CAs have a so-called closed active site (at pH < 8.3), in which the fourth zinc ligand is an aspartate residue, and thus these enzymes are devoid of CO2 hydrase activityCitation21–23. However, at pH values > 8.3, the aspartate is involved in a hydrogen bond with an adjacent Arg residue, and an incoming water molecule/hydroxide ion replaces the aspartate as a zinc ligand, providing an open active site and thus a catalytically active enzymeCitation21–23.
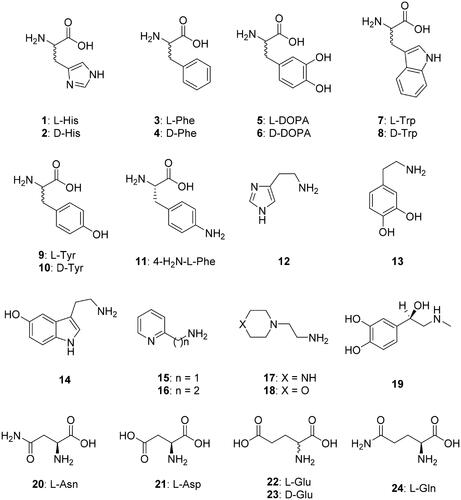
Table 1. Activation of hCA isozymes I, II, EcoCAβ and TvaCA1 with L-Trp, measured at 25 °CCitation15.
Table 2. Activation constants of hCA I, hCA II and the bacterial enzyme EcoCAβ (E. coli) and the protozoan TvaCA1 (T. vaginalis) with amino acids and amines 1–24, by a stopped-flow CO2 hydrase assayCitation15.
