Figures & data
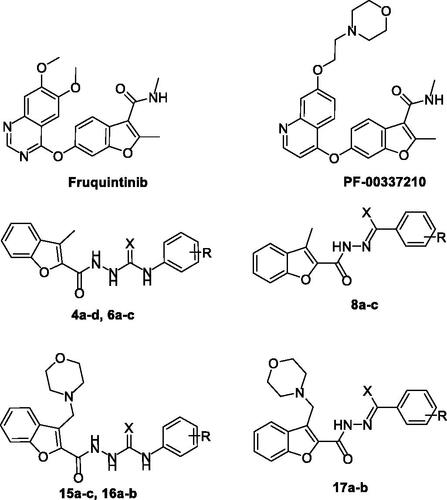
Figure 1. Chemical structures of benzofurans Fruquintinib and PF-00337210, as well as structures of target 2-methylbenzofurans (4a–d, 6a–c and 8a–c) and 3-(morpholinomethyl)benzofurans (15a–c, 16a–b and 17a–b).
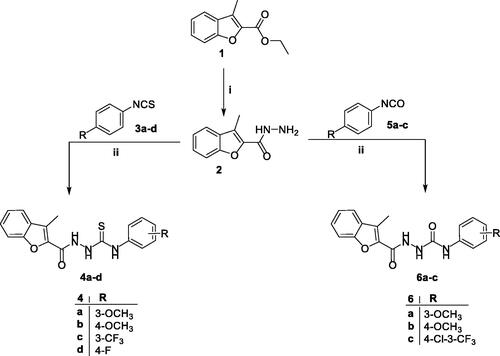
Scheme 1. Synthesis of target 2-methylbenzofurans 4a–d and 6a–c; reagents and conditions: (i) NH2NH2.H2O/isopropyl alcohol/reflux 2 h and (ii) dry toluene/reflux 7 h.
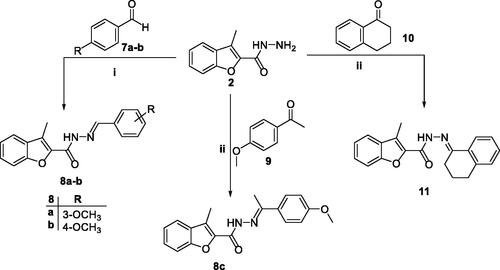
Scheme 2. Synthesis of target 2-methylbenzofurans 8a–c and 11; reagents and conditions: (i) Ethanol/Cat. Acetic acid/reflux 3 h, (ii) ethanol/cat. Acetic acid/reflux 7 h.
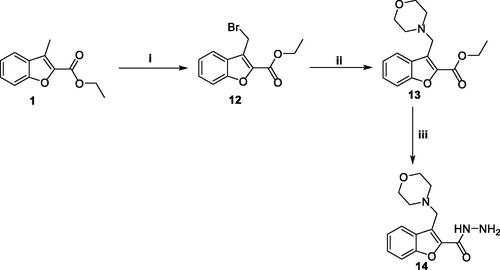
Scheme 3. Synthesis of key intermediate 3-(morpholinomethyl)benzofuran-2-carbohydrazide 14; Reagents and conditions: (i) NBS/carbon tetrachloride/dibenzoyl peroxide/reflux 16 h, (ii) Morpholine/Acetonitrile/K2CO3/KI/reflux 8 h, and (iii) NH2NH2.H2O/isopropyl alcohol/reflux 2 h.
Scheme 4. Synthesis of target 3-(morpholinomethyl)benzofurans 15–18; reagents and conditions: (i) dry toluene/reflux 7 h and (ii) ethanol/cat. Acetic acid/reflux 3 h.
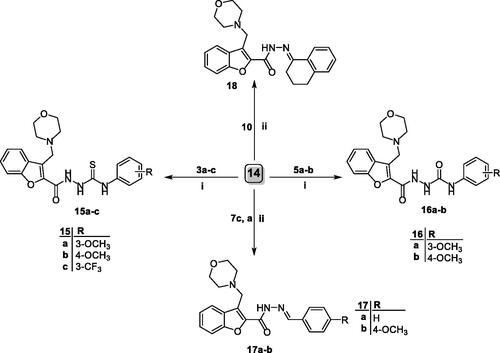
Figure 2. Effect of 3-methylbenzofuran derivative 4b on the phases of cell cycle of A549 cells, and effect of 3-(morpholinomethyl)benzofuran derivatives 15a and 16a on the phases of cell cycle of NCI-H23 cells.
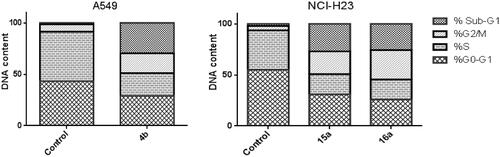
Figure 3. Effect of 3-methylbenzofuran derivative 4b on the percentage of annexin V-FITC-positive staining in Non-small cell lung cancer A549 cells.
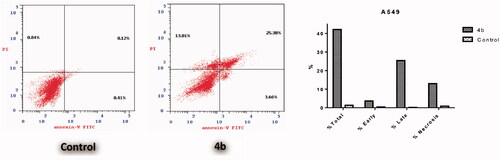
Figure 4. Effect of 3-(morpholinomethyl)benzofuran derivatives 15a and 16a on the percentage of annexin V-FITC-positive staining in Non-small cell lung cancer NCI-H23 cells.
Table 1. Cytotoxic impact against non-tumorigenic human lung WI-38 cell line, as well as mean tumour selectivity index (S.I.) (WI-38/A549 and NCI-H23).
Table 2. In vitro anti-proliferative activity of target 3-methylbenzofurans (4a–d, 6a–c, 8a–c and 11) and 3-(morpholinomethyl)benzofurans (15a–c, 16a–b, 17a–b and 18) against lung A549 and NCI-H23 cancer cell lines. 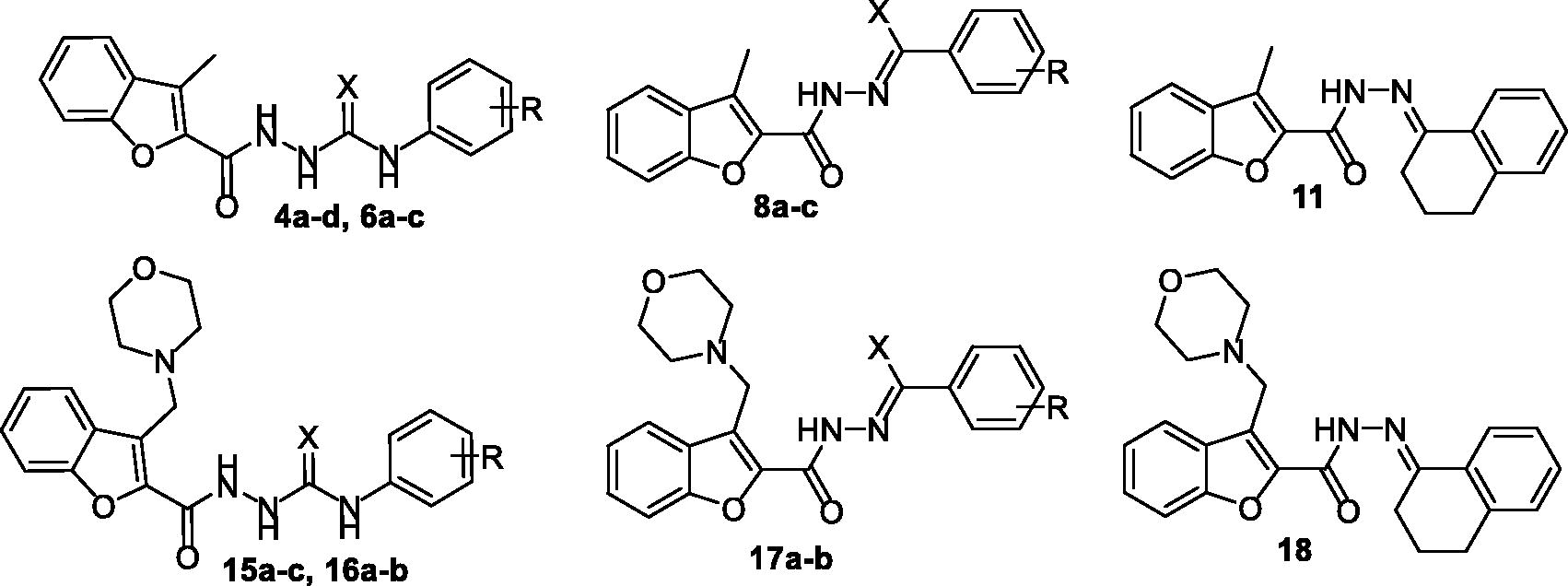
Table 3. Inhibitory activity of target benzofurans 4b, 15a and 16a against VEGFR-2.
Table 4. Anti-tubercular activity of the target compounds 4b, 15a and 16a.
Figure 5. 2D (A) and 3D (B) interactions of 3-methylbenzofuran derivative 4b within VEGFR-2 binding site (PDB: 4ASD).
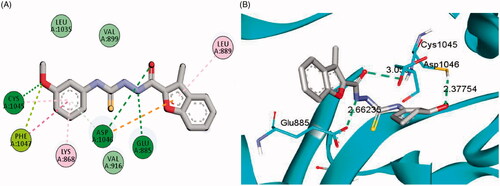
Figure 6. 2D (A) and 3D (B) interactions of 3-(morpholinomethyl)benzofuran derivative 15a within VEGFR-2 binding site (PDB: 4ASD).
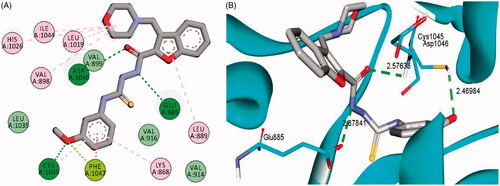
Figure 7. 2D (A) and 3D (B) interactions of 3-(morpholinomethyl)benzofuran derivative 16a within VEGFR-2 binding site (PDB: 4ASD).
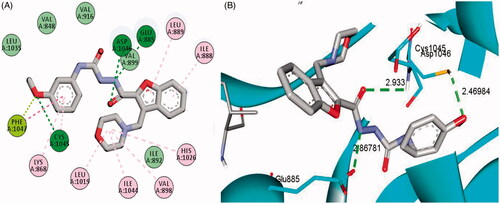
Table 5. The detailed bonding interactions and binding scores for the benzofurans 4b, 15a and 16a.
