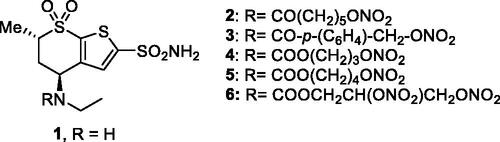Figures & data
Figure 1. The multitargeting approach with a dual/multiple ligand, schematically shown as the triple arrow, which acts on multiple (in this case three) different targets, represented as A, B and C.
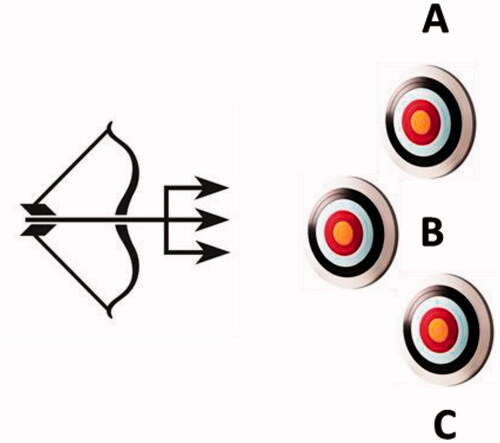
Figure 3. Binding of sulphonamide–NO donors to hCA II as determined by X-ray crystallographic experiments. (A) Compound 6 (PDB 3K2F) in magenta, and (B) sulphonamide 8 (PDB 3NI5) is shown in green. The zinc ion from the CA active site is shown as a gold sphere, being coordinated by three His residues (His 94, 96 and 119 – not numbered in the figure for the sake of simplicity) and the fourth metal ion ligand being the deprotonated sulphonamide from the inhibitor. The other amino acid residues involved in contacts with the inhibitors are also highlighted.
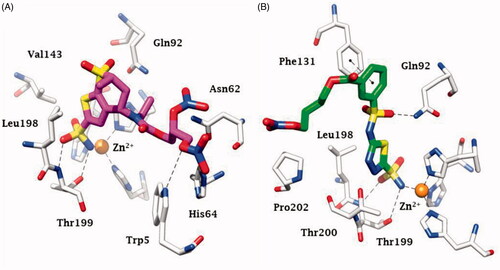
Figure 4. Chemical structure of aromatic/heterocyclic sulphonamides incorporating NO-donating moieties of the aliphatic nitrate ester type, 7–9.
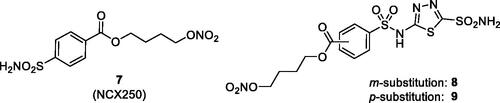
Figure 7. (A) Sulphonamide CAI–β-blocker hybrids of types 13–16. (B) X-ray crystal structure for the adduct of 14 with hCA II: the zinc ion (gray sphere) with its three protein ligands (His94, 96, 119) are evidenced. The inhibitor 14 (blue sky) is coordinated with the deprotonated sulphonamide moiety to the metal ion, and its scaffold participates in a range of favourable interactions with active site residues, such as Thr199, Leu198, Phe131, Val135. Hydrogen bonds are shown as dashed lines.
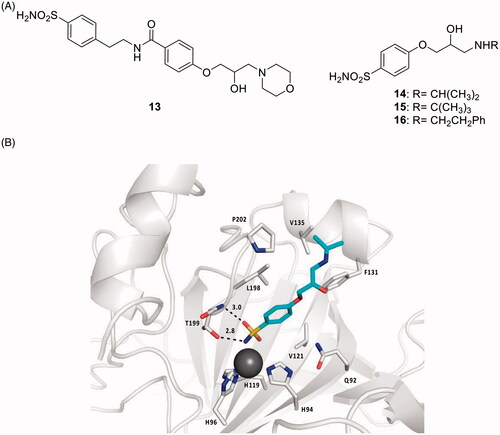
Figure 8. General strategy for obtaining CAI–CORM hybrids 18 based on the hexacarbonyl dicobalt(II) complexes.
Figure 9. CAI–CORM hybrids of types 19–22 incorporating sulphonamide, 7-hydroxycoumarin, 7-aminocoumarin and 6-hydroxysulphocoumarin as scaffolds with CA inhibitory properties and hexacarbonyl dicobalt(II) as CO releasing fragment.
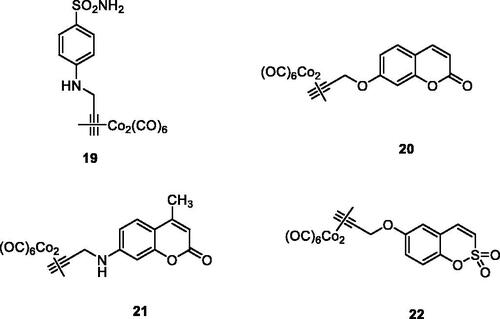
Figure 10. General structures of the COX inhibitor-CAI hybrids A and B, and several coumarin–NSAIDs hybrids of type 23–27.
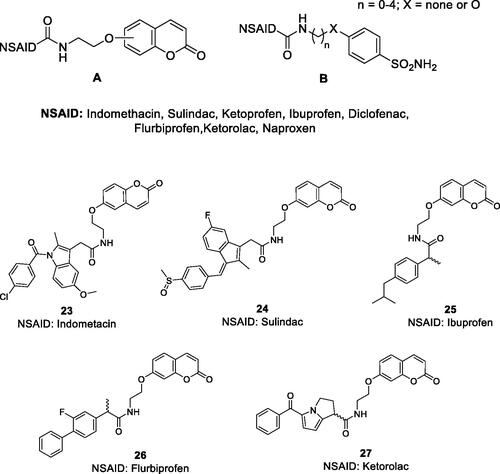
Figure 11. General strategy for hybridisation of CAIs with cytotoxins in compounds of type C. CA IX/XII–cytotoxic agent hybrids, incorporate toxic payloads 28–32, whereas the CA inhibitory warheads may be benzenesulphonamide or 1,3,4-thiadiazole-2-sulphonamide derivatives (although coumarins and other CAI chemotypes can be used). The linker is usually a tetrapeptide incorporating water-solubilizing residues (Asp, Arg, Cys).
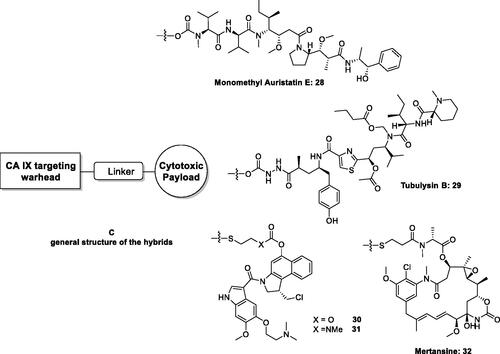
Figure 12. DHA–CAI hybrids incorporating sulphonamide (33 and 35) and coumarin moieties (34) as CA inhibitory fragment. In compounds 35R = sulfamoyl, in meta or para positions. The linkers may be 1,2,3-triazole based moieties obtained by click chemistry, –O–(CH2)n–O–, n = 2–4, etc.
Figure 13. Multitargeting compounds incorporating the kinase inhibitory fragment from erlotinib and sulphonamide CAIs of type 36 and compounds 37 and 38 targeting CAs, COX-2, and 15-LOX.
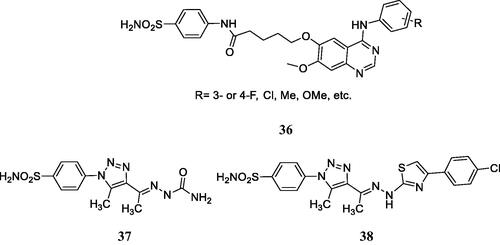
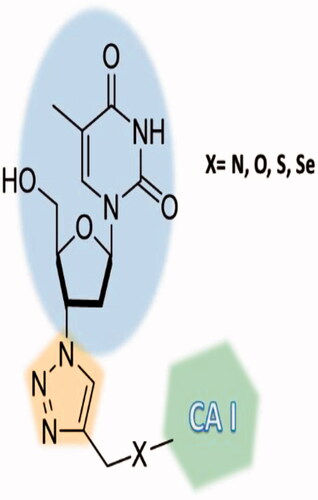
Figure 15. Telomerase-CAI hybrids 39 (a–u) incorporating sulphonamide, coumarin, sulphocoumarin fragments with CA inhibitory action, AZT as telomerase inhibitor and various linkers (ether, thioether, amine, amide, ester, selenoether, carbamate, etc.).
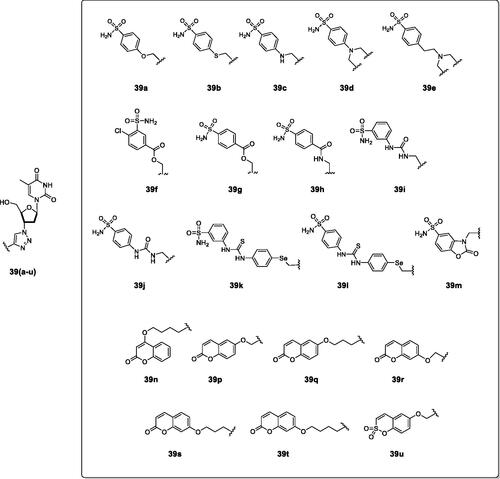
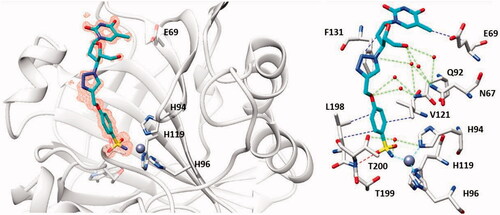
Figure 16. The adduct of hCA II with 39a as determined by X-ray crystallography. Left: hCA II active site, with the zinc ion (gray sphere) and its histidine ligands (His94, 96 and 119) and the inhibitor showed with its electronic density. Right: detailed interactions in which the sulphonamide 39a participates with amino acid residues from the enzyme active site. Red spheres are water molecules, dashed lines represent hydrogen bonds. Residues involved in the binding are evidenced.