Figures & data
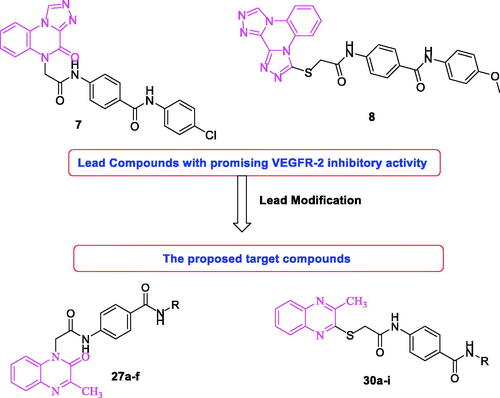
Figure 1. Some clinically used VEGFR-2 inhibitors as well as quinoxaline derivatives having VEGFR-2 inhibitory actions.
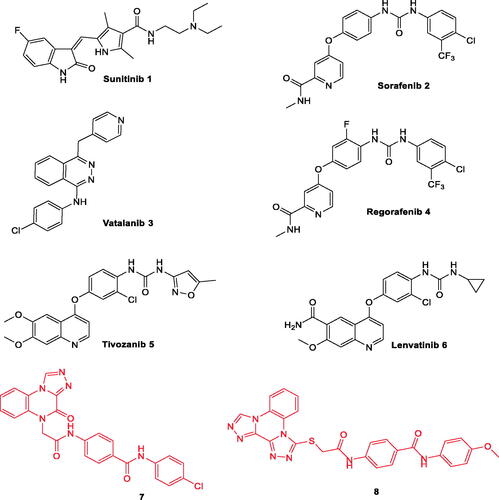
Table 1. In vitro cytotoxicity of the synthesised compounds against MCF-7 and HepG2 cell lines, their VEGFR-2 inhibitory activities on cancer HepG2 cell line, and cytotoxicity for compounds 27a and 30f against normal HepG2cell line.
Figure 3. Correlation of cytotoxicity of the synthesised compounds on the two tested cell lines; MCF-7 and HepG2 showing higher sensitivity against HepG2 than MCF-7 cell line.
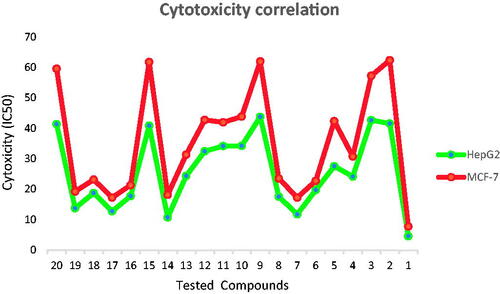
Figure 4. Flow cytometric analysis of cell cycle phases after treatment with compound 27a. (A) histograms showing the cell cycle distribution of control and treated cells. (B) Column graphs showing the percentage of cells in each phase of the cell cycle.
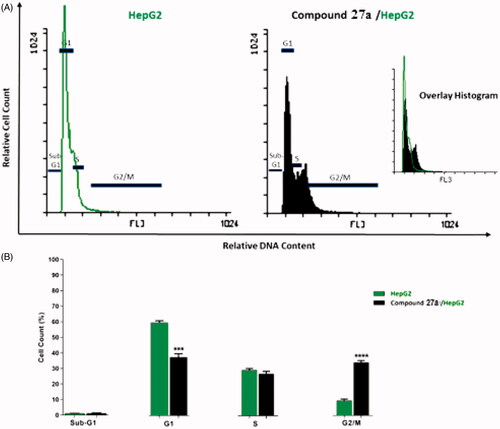
Table 2. Effect of compound 27a on cell cycle progression in HepG2 cells.
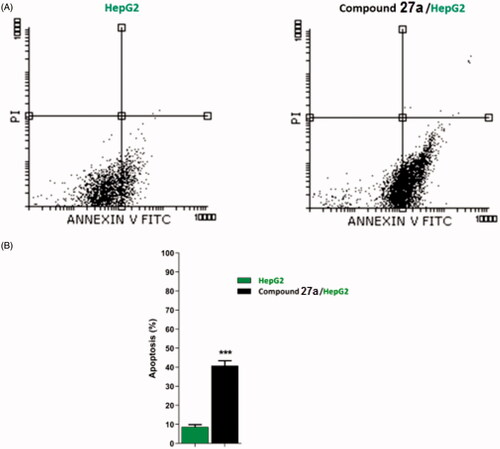
Table 3. Stages of the cell death process in HepG2 cells after treatment with compound 27a.
Figure 6. The immunoblotting of caspase-3, caspase-9, BAX, and Bcl-2 (normalised to β-actin). (A) Representative Western blot images show the effect of compound 27a (at its IC50 concentration) on the expression levels of BAX, Bcl-2, active caspases-9, and active caspases-3 proteins in HepG2 cells.
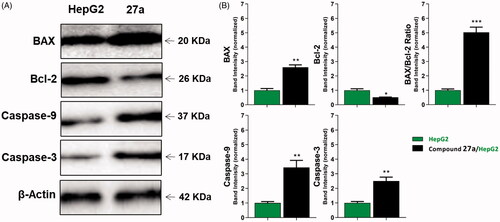
Table 4. Effect of compound 27a on the levels of active caspases-3, active caspases-9, BAX, and Bcl-2 proteins in HepG2 cells treated for 24 h.
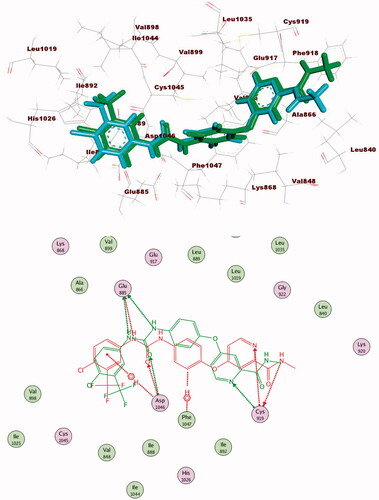
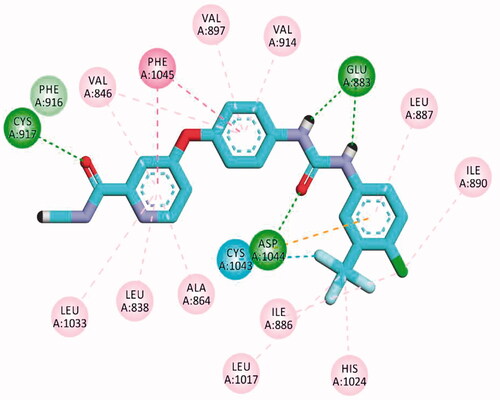
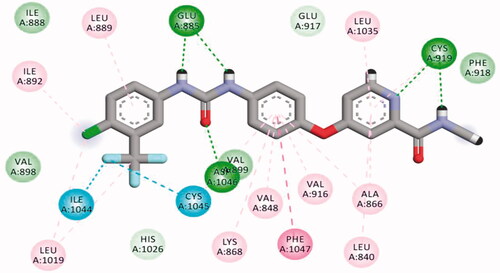
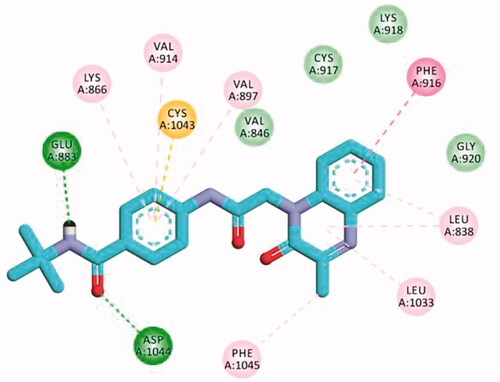
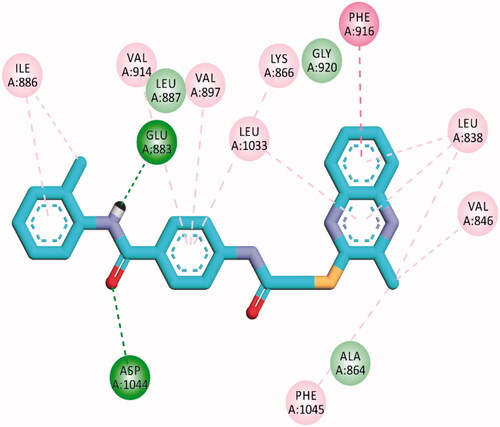
Table 5. The calculated ΔG (binding free energies) of the synthesised compounds and reference drug against VEGFR-2 (PDB ID; 2OH4 and 4ASD) (ΔG in Kcal/mole).
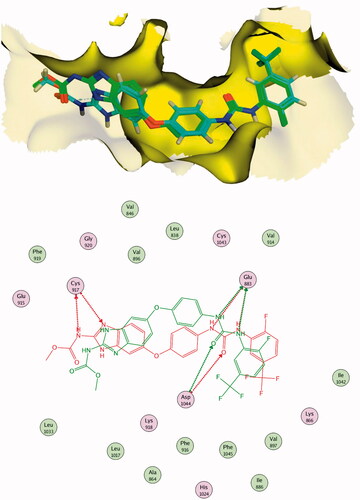
Figure 7. Alignment of the co-crystallised pose and the re-docked pose of the same ligand (PDB ID; 2OH4).