Figures & data
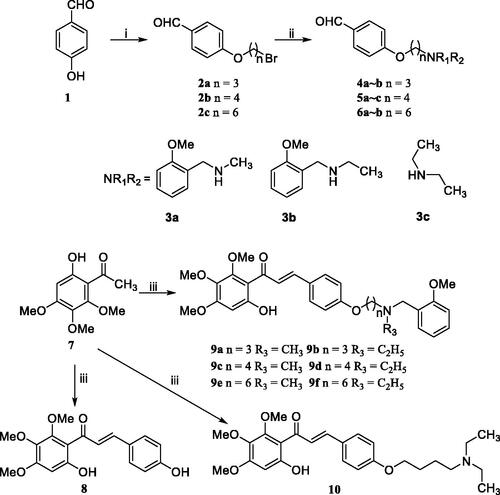
Scheme 1. Synthesis of target chalcone-Vitamin E-donepezil hybrids 8, 9a–f, and 10. Reaction conditions: (i) Br(CH2)nBr, K2CO3, CH3CN, 65 °C, 6–10 h; (ii) NHR1R2 (3a–c), K2CO3, CH3CN, refluxed, 6–8 h; (iii) 1, 4a–b, 5a–c, and 6a–b, 50% KOH, r.t., 3–4 days.
Scheme 2. Synthesis of target chalcone-Vitamin E-donepezil hybrids 16 and 17a–f. Reagents and conditions: (i) chloromethyl methyl ether, (i-Pr)2EtN, acetone, 50 °C, 6–8 h; (ii) 4a–b, 5a–b, and 6a–b, 50% KOH, r.t., 3–4 days; (iii) 1, 50% KOH, r.t., 3–4 days; (iv) 10% HCl, room temperature, overnight.
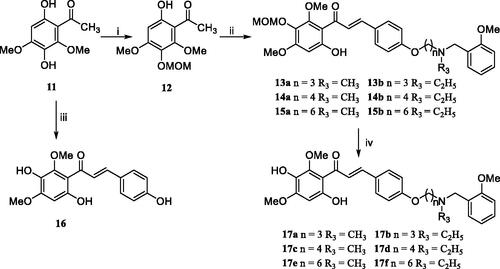
Table 1. AChE and BuChE inhibitory potency and oxygen radical absorbance capacity (ORAC, Trolox equivalent) by chalcone-Vitamin E-donepezil hybrids, Vitamin E, and donepezil.
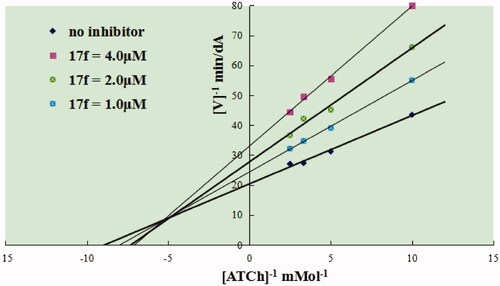
Figure 3. Compound 17f (green stick) interacted with AChE (PDB code: 1eve) (A) Interactions in the active site. (B) 3D docking model. (C) 2D docking model.
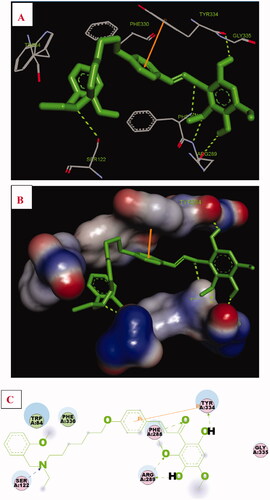
Figure 4. (A) RMSD analysis of compound 17f (green stick) in AChE (PDB code: 1eve). (B) The docking model for 17f into the protein crystal structure of AChE (PDB code: 1eve).
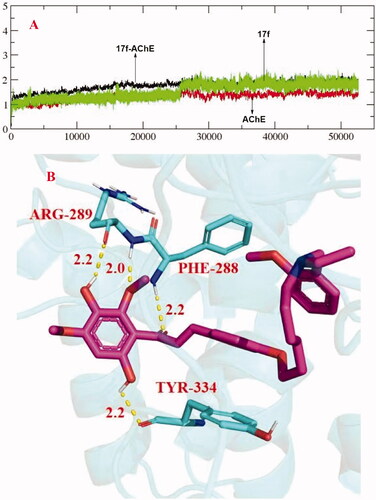
Table 2. The results of propidium iodide displacement assay and inhibition of huAChE-induced Aβ aggregation towards compound 17f and donepezil.
Table 3. Inhibition and disaggregation potency of Aβ1–42 aggregation by donepezil, curcumin, and chalcone-Vitamin E-donepezil hybrids.
Figure 5. TEM images analysis of self-medicated Aβ1–42 aggregation by curcumin and compound 17f. (A) Inhibition experiments. (B) Disaggregation experiments.
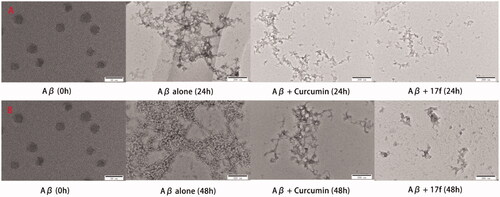
Figure 6. The UV spectrum of compounds 9d and 17f alone or in the presence of CuCl2, AlCl3, ZnCl2, and FeSO4. The final concentration was 37.5 μM.
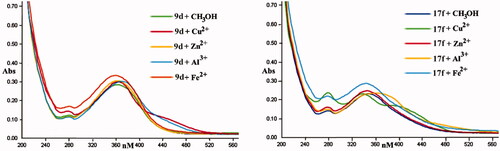
Figure 7. Determination of the stoichiometry of complex compound-Cu2+ by using the molar ratio method. The final concentration of compounds 9d and 17f was 37.5 μM, with ascending amounts of CuCl2.
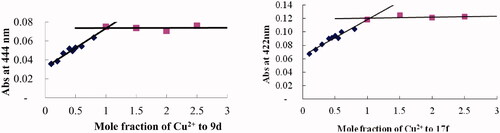
Figure 8. TEM images analysis of Cu2+-mediated Aβ1–42 aggregation by curcumin and compound 17f. (A) Inhibition experiments. (B) Disaggregation experiments.
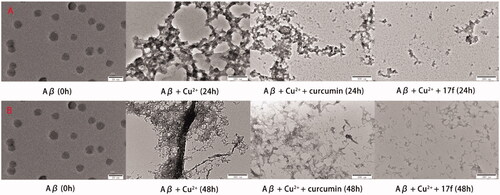
Figure 9. Docking studies of 17f with Aβ1–42 (PDB ID: 1BA4). (A) Cartoon model. (B) Interactions in the C-terminus of the active site. (C) 2D docking model.
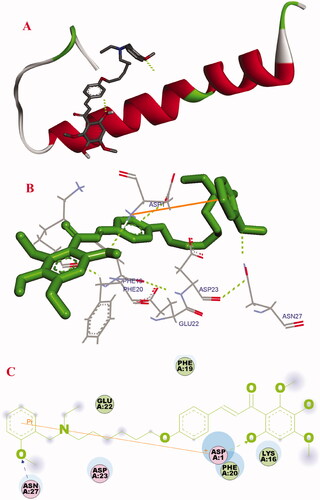
Table 4. Inhibition potency of huMAO-A and huMAO-B) and selectivity index (SI) values of clorgyline, rasagiline, iproniazid, and chalcone-Vitamin E-donepezil hybrids.
Figure 10. The interactions between compound 17f (green stick) and the residues of the active site in huMAO-B (PDB code: 2V60).
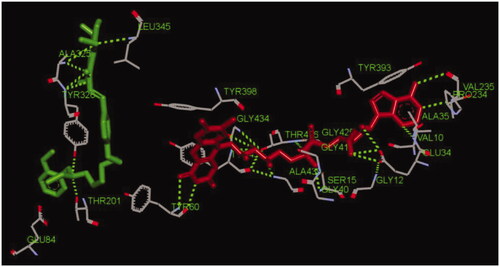
Figure 11. (A) Cytotoxicity of 17f in PC12 cells. (B) Attenuation of H2O2-induced PC12 cell injury by compound 17f was tested using MTT assay. (C) The LDH activity of compound 17f on H2O2-induced PC12 cell injury was evaluated using LDH assay. Three independent experiments were carried out. Data were expressed as mean ± SD and percentage of control value. ##p < 0.01 vs. control; **p < 0.01, *p < 0.05 vs. H2O2 group. VE: Vitamin E.
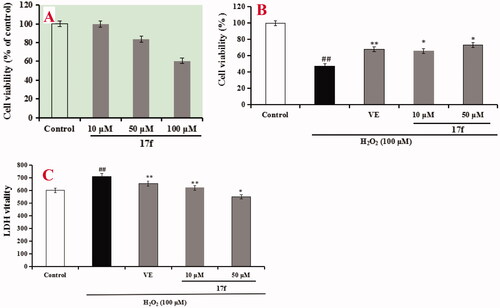
Table 5. The predictive penetration of 17f by PAMPA-BBB assay.