Figures & data
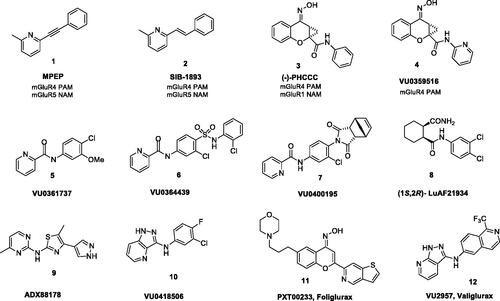
Figure 1. Chemical structures of various classes of mGlu4 PAMs. Compounds 1 and 2 were the first ligands identified as PAMs for mGlu4 receptor, 11 is currently in phase II clinical trials, and 12 has advanced as a preclinical development candidate.
Scheme 1. Reagents and conditions: (a) NH2OH·HCl, NaOHaq, EtOH, reflux, 1–5 h; (b) R1COCl, toluene, K2CO3, MW 170 °C, 10 min; (c) Fe, CH3COOH, EtOH, water, 60 °C, 1–2 h or SnCl2, 5 N HCl, EtOH, reflux, 2 h; (d) R2COCl, py, rt, overnight.
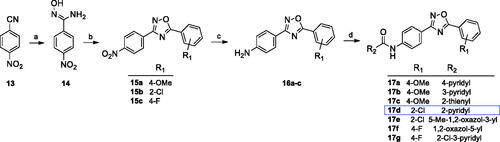
Scheme 2. Reagents and conditions: (a) NH2OH·HCl, NaOHaq, EtOH, reflux, 1–5 h; (b) 4-nitrobenzoyl chloride, toluene, K2CO3, MW 170 °C, 10 min; (c) Fe, CH3COOH, EtOH, water, 60 °C, 1–2 h or Raney Ni, NH2-NH2 aq, MeOH/THF, 60 °C, 30 min; (d) R2COCl, py, rt, overnight.
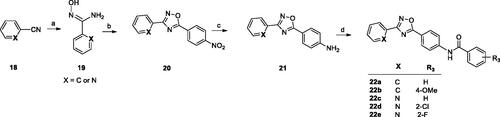
Scheme 3. Reagents and conditions: (a) py, rt, overnight; (b) NH2OH·HCl, NaOHaq, EtOH, reflux, 1–5 h; (c) R2COCl, toluene, K2CO3, MW 170 °C, 10 min or rt, 30 min than reflux, 6 h.
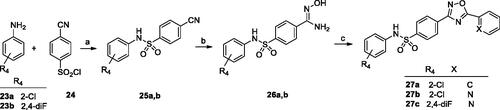
Table 1. In vitro mGlu4 receptor PAM activity of 1,2,4-oxadiazole derivatives.
Figure 4. The activity of compounds 34, 37, 49, 52, 60, 62 and reference drugs MMPIP (a selective NAM of mGlu7 receptor) and AZ12216052 (a PAM of mGlu8 receptor) tested at a concentration of 10 μM in the cAMP accumulation assay in cells expressing mGlu7 and mGlu8 receptors. The percent activity refers to two extreme FRET signals: 0% corresponds to forskolin treatment alone and 100% to maximal FRET signal for the control cells (without any treatment). A/D – agonistic activity; B/E – PAM activity measured in the presence of L-Glu at the EC20 concentration (0.1 mM for mGlu7 receptor, and 1 μM for mGlu8 receptor); C/F – NAM activity measured in the presence of L-Glu at the EC80 concentration (1 mM for mGlu7 receptor, 10 μM for Glu8 receptor).
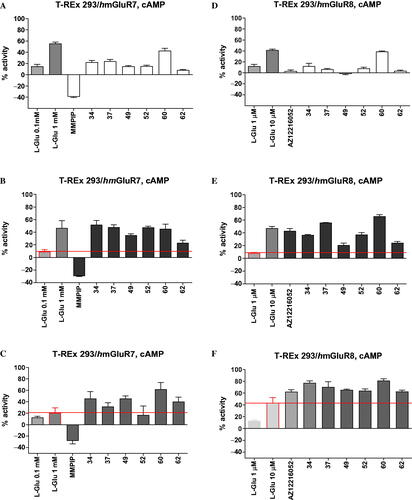
Figure 5. The time course of 52 after i.p. administration in mice: (A) plasma and (B) brain concentration-time profiles.
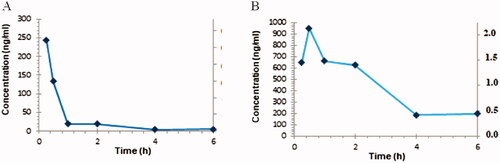
Table 2. Pharmacokinetic parameters in the mouse plasma and brain after a 10 mg/kg i.p. dose of 52.
Figure 6. The effects of compounds 52 and 62 on (A, B) stress-induced hyperthermia and (D, E) DOI-induced HTR. Doses are indicated as mg/kg. Bars represent the mean ± SEM ***p < 0.001 vs. vehicle. Compounds 52 and 62 at all doses significantly reduced the SIH response: 52 [F(3.34) = 9.35; p < 0.001], 62 [F(3.36)=17.12; p < 0.001]; and DOI-induced HTR: 52 [F(3.36) = 28.14; p < 0.001]), 62 [F (3.16) = 38.24; p < 0.001]. The control drugs are presented in panels C (diazepam) and F (clozapine). ***p < 0.001 and **p < 0.01.
![Figure 6. The effects of compounds 52 and 62 on (A, B) stress-induced hyperthermia and (D, E) DOI-induced HTR. Doses are indicated as mg/kg. Bars represent the mean ± SEM ***p < 0.001 vs. vehicle. Compounds 52 and 62 at all doses significantly reduced the SIH response: 52 [F(3.34) = 9.35; p < 0.001], 62 [F(3.36)=17.12; p < 0.001]; and DOI-induced HTR: 52 [F(3.36) = 28.14; p < 0.001]), 62 [F (3.16) = 38.24; p < 0.001]. The control drugs are presented in panels C (diazepam) and F (clozapine). ***p < 0.001 and **p < 0.01.](/cms/asset/152fc2db-26eb-4466-9308-93fef34c5ca8/ienz_a_1998022_f0006_b.jpg)