Figures & data
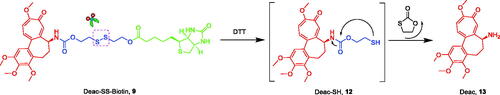
Figure 3. Reduction-sensitive drug release mechanism of Deac-SS-Biotin triggered by DTT (Glutathione mimetic).
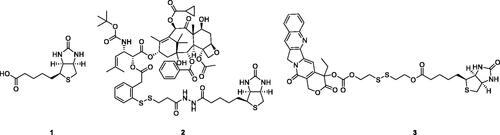
Scheme 1. Reagents and conditions: (a) 4-nitrophenyl carbonochloridate, Et3N, THF, rt., 6 h; (b) THF, rt., 4 h; (c) 1, DCC, DMAP, rt., 20 h; (d) HATU, Et3N, rt., 8 h; (e) anhydrides, NMM, DMSO, rt., 45 min; (f) (i) SOCl2, MeOH, rt., overnight; (ii) NH2NH2, rt., 17 h; (g) EDCI, HOBt, DMAP, Et3N, rt., 10 h.
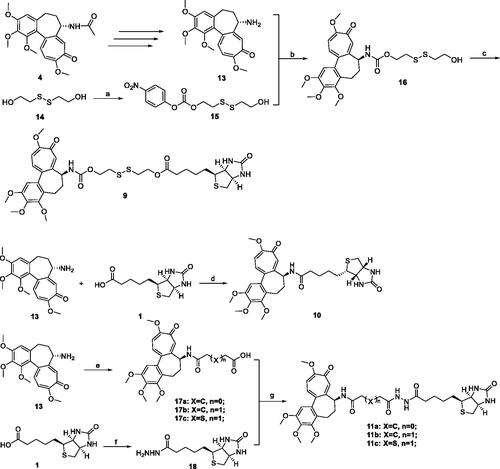
Figure 4. Stabilities of Deac-SS-Biotin (9, 5 µM) in water, and PBS cell culture fluid were investigated by HPLC analysis. (A) HPLC analysis of Deac-SS-Biotin (9) in water after incubation for and 72 h; (B) HPLC analysis of Deac-SS-Biotin (9) in PBS after incubation for and 72 h; (C) HPLC analysis of Deac-SS-Biotin (9) in cell culture fluid after incubation for and 72 h.
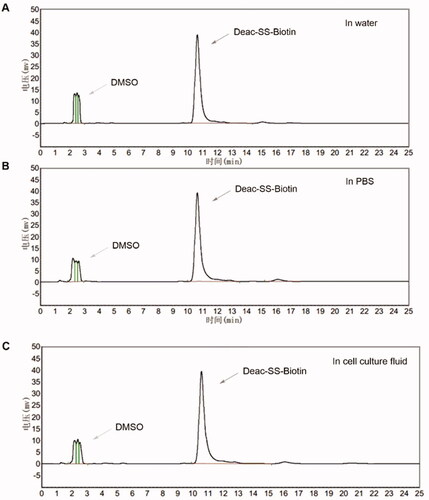
Figure 5. In vitro release of Deac from prodrugs. (A) Release profiles of Deac from Deac-SS-Biotin (9, 5 μM) with 0 μM, 5 μM, 10 μM and 20 μM DTT in PBS (pH 7.2–7.4) (n = 3). (B) Release profiles of Deac from Deac-Biotin (10, 5 μM) with 0 μM, 5 μM, 10 μM and 20 μM DTT in PBS (pH 7.2–7.4) (n = 3). (C) HPLC spectra of Deac-SS-Biotin (9, 5 μM) with 10 μM DTT at 0 and 24 h.
Table 1. Antiproliferative activity of all compounds.
Figure 6. In vitro cytotoxicity of biotin, Deac (13), and Deac-SS-Biotin (9) in A549 cells. Cytotoxicity of biotin, Deac + biotin, and Deac-SS-Biotin + biotin in A549 cells (n = 3).
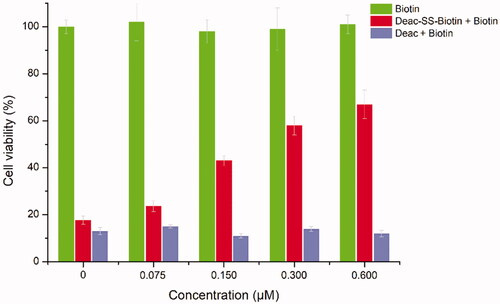
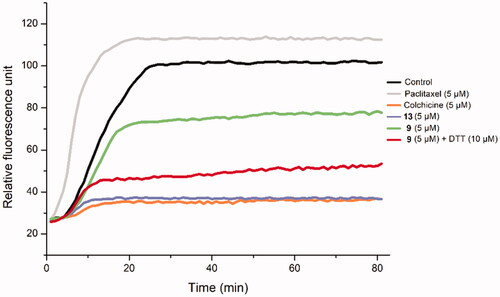
Figure 7. The effect of Deac-SS-Biotin (9) on tubulin polymerisation. The tubulin had been pre-incubated for 1 min with Deac (13) at 5 µM, Deac-SS-Biotin (9) at 5 µM, Deac-SS-Biotin (9) at 5 µM and DTT at 10 µM, Deac (13) at 5 µM, Colchicine at 5 µM, Paclitaxel at 5 µM or vehicle DMSO at room temperature before GTP was added to start the tubulin polymerisation reactions. The reaction was monitored at 37 °C.