Figures & data
Figure 1. (Left) X-ray crystallography of FLT3 kinase (PDB:4RT7); (Right) The region and amino acid residues in which FLT3 mutations commonly occur; (Blue) Juxtamembrane; (Red) Activation loop of the kinase domain.
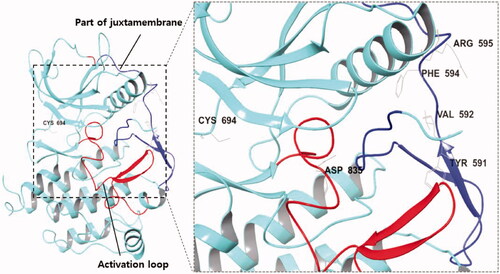
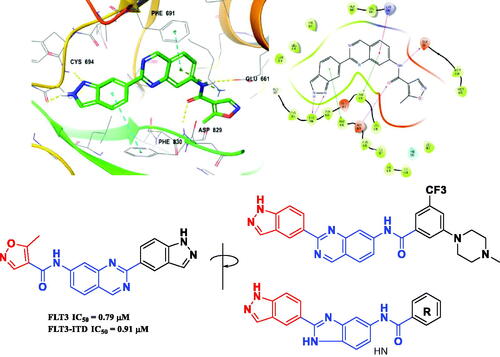
Figure 3. Docking structures of a quinazoline derivative with an indazole fragment in FLT3 (PDB: 4RT7) and Design of a benzimidazole analog with an indazole moiety from studies on the binding mode of a previous FLT3 inhibitor.
Scheme 1. Synthesis of N-(2-(1H-indazol-6-yl)-1H-benzo[d]imidazol-5-yl)benzamide derivatives. (i) 3,4-dihydro-2H-pyran, Pyridinium p-toluenesulfonate, µW, 50 °C, 5 h; (ii) LiAlH4 in THF, THF, 0 °C; (iii) Dess-Martin periodinane, MC/THF = 1:1, rt; (iv) 4-Nitrobenzene-1,2-diamine, NH4Cl, EtOH, reflux; (v) H2, Pd/C, EtOH; (vi) Benzoic acid, EDC, HOBt, TEA, THF, rt; (vii) 20% TFA, CH2Cl2 or 5% HCl in EtOH.
![Scheme 1. Synthesis of N-(2-(1H-indazol-6-yl)-1H-benzo[d]imidazol-5-yl)benzamide derivatives. (i) 3,4-dihydro-2H-pyran, Pyridinium p-toluenesulfonate, µW, 50 °C, 5 h; (ii) LiAlH4 in THF, THF, 0 °C; (iii) Dess-Martin periodinane, MC/THF = 1:1, rt; (iv) 4-Nitrobenzene-1,2-diamine, NH4Cl, EtOH, reflux; (v) H2, Pd/C, EtOH; (vi) Benzoic acid, EDC, HOBt, TEA, THF, rt; (vii) 20% TFA, CH2Cl2 or 5% HCl in EtOH.](/cms/asset/a652d3a7-3825-48b7-a442-b282b33ef4c0/ienz_a_2020772_sch0001_b.jpg)
Scheme 2. Synthesis of 1–(2-(1H-indazol-6-yl)-1H-benzo[d]imidazol-5-yl)-3-phenylurea derivatives. (i) (1) 4-Nitrophenyl chloroformate, DIPEA, THF, 0 °C; (2) RNH2, THF, 50 °C; (ii) 20% TFA, CH2Cl2.
![Scheme 2. Synthesis of 1–(2-(1H-indazol-6-yl)-1H-benzo[d]imidazol-5-yl)-3-phenylurea derivatives. (i) (1) 4-Nitrophenyl chloroformate, DIPEA, THF, 0 °C; (2) RNH2, THF, 50 °C; (ii) 20% TFA, CH2Cl2.](/cms/asset/3d5cb562-b296-4304-9fe9-37c1d74a06e2/ienz_a_2020772_sch0002_b.jpg)
Table 1. Enzymatic activities of N-(2-(1H-indazol-6-yl)-1H-benzo[d]imidazol-5-yl)benzamide and 1–(2-(1H-indazol-6-yl)-1H-benzo[d]imidazol-5-yl)-3-phenylurea derivatives
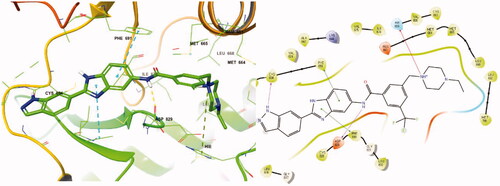
Figure 4. Docking structure of compound 8a at the active site of FLT3 (Left) and at the hydrophobic pocket adjacent to the ATP-binding site (Right).
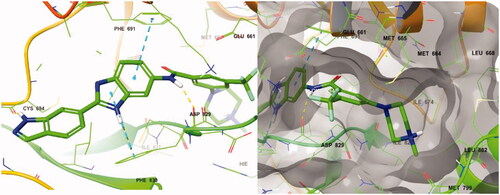
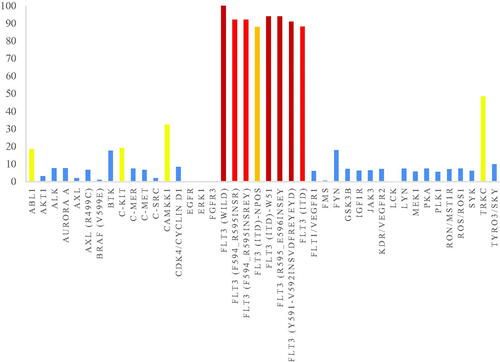
Table 2. Enzymatic inhibitory activities of compound 8r against FLT3 mutants.
Table 3. IC50 for the enzymatic inhibitory activity of compound 8r.
Figure 6. Docking structure of 8r in FLT3 (PDB: 4RT7)Citation26.