Figures & data
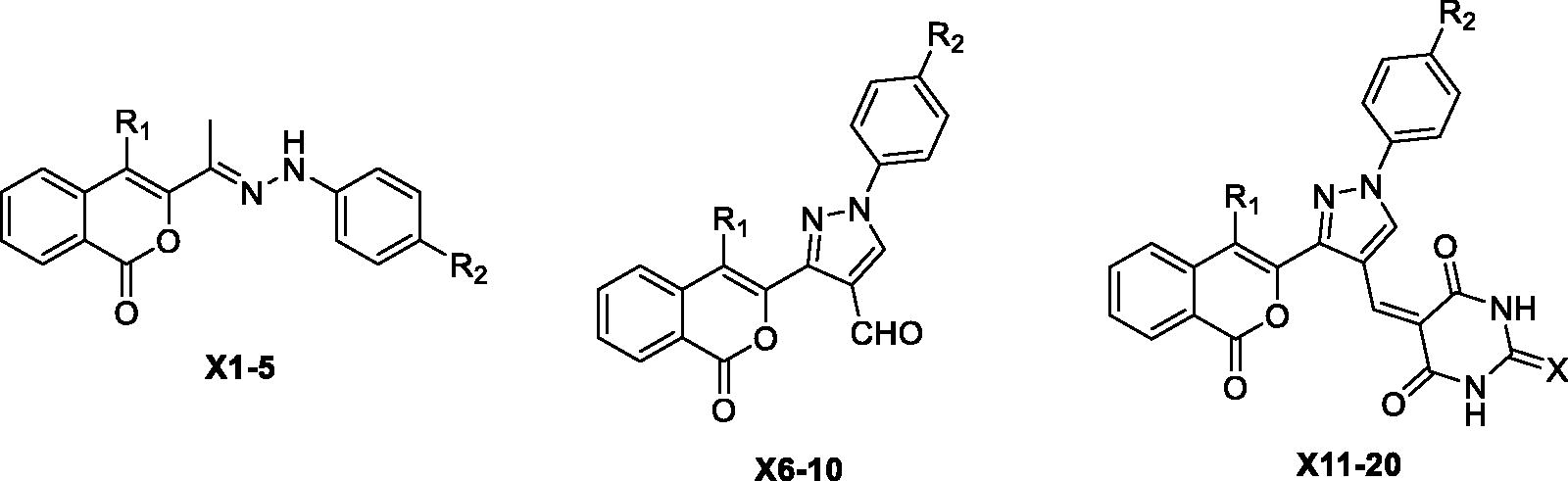
Scheme 1. General synthetic route for the synthesis of the isocoumarin-substituted compounds X(1-20). Reagent and conditions: (i) chloroacetone, TEA, 170 °C, (ii) substituted phenylhydrazine hydrochloride, EtOH, sodium acetate, 2 h reflux, (iii) DMF/POCl3, 0–5 °C, then 3 h reflux, (iv) barbituric acid/2-thiobarbituric acid, acetic acid.
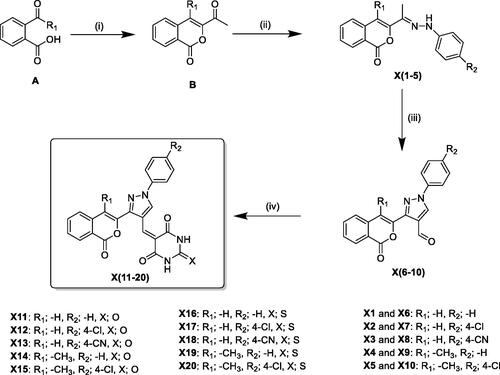
Table 1. Inhibition data of human CA I, II, IX and XII with compounds X1-20 and the standard sulphonamide inhibitor acetazolamide (AAZ) by a stopped-flow CO2 hydrase assayCitation7.