Figures & data
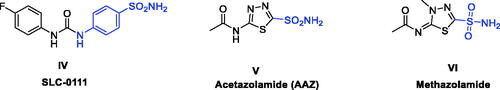
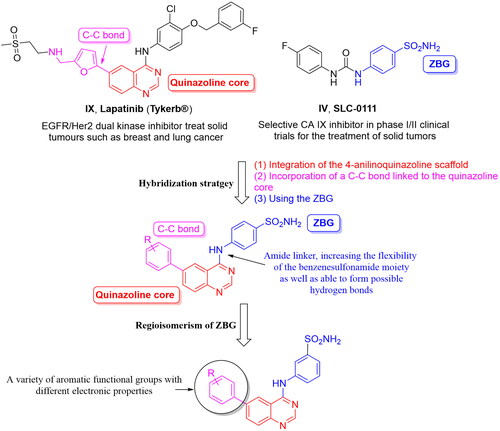
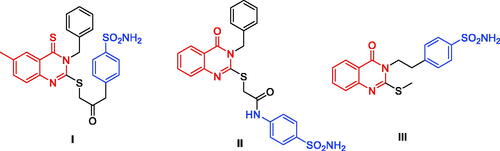
Figure 1. Chemical structures of potent quinazoline-based analogues as carbonic anhydrase inhibitors possessing primary sulphonamide (ZBG) moiety.
Scheme 1. Reagents and conditions: (i) DMF-DMA, 100 °C, 1.5 h; (ii) appropriate aniline, GAA, reflux, 2 h; (iii) suitable boronic acid derivative, Pd(amphos)C12, T3P, dioxane, 80 °C, 2 h (For 4a–l, R5 and R6 are described in detail in ).
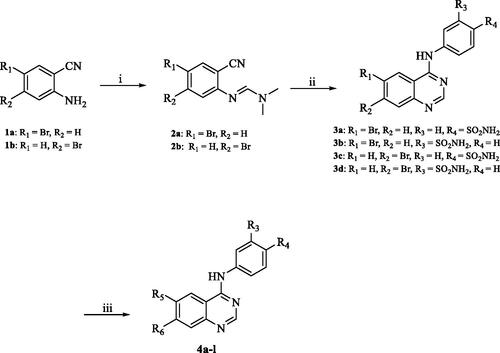
Table 1. The inhibitory activity (Ki) of the synthesised hybrids against hCA I, II, IX and XII isoforms
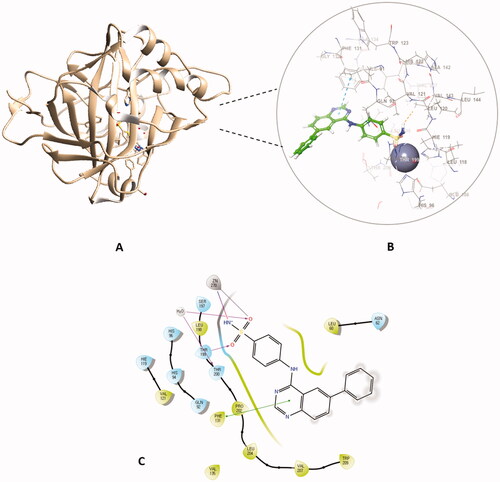
Figure 6. The binding patterns of compound 4f inside the binding cavity of hCAI and IX. (A) 3D model of the crystal structure of hCA I with compound 4f. (B) 2D interaction pattern of compound 4f with hCA I binding cavity. (C) 3D model of the crystal structure of hCA IX with compound 4f. (D) 2D interaction pattern of compound 4f with hCA IX binding cavity.
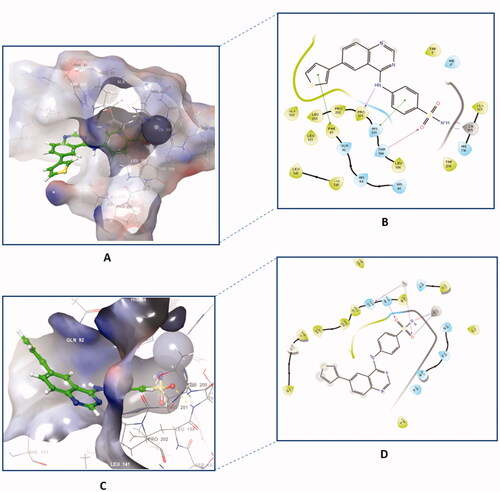
Figure 7. The 2D interaction patterns of compound 4f with hCA II (A) and XII (B). Favourable interactions are colour coded as follows: purple‒hydrogen bond, grey‒metal bond, red‒π-cation interaction, green‒π‒π stacking interactions. The light green lines represent weak van der Waals interactions.
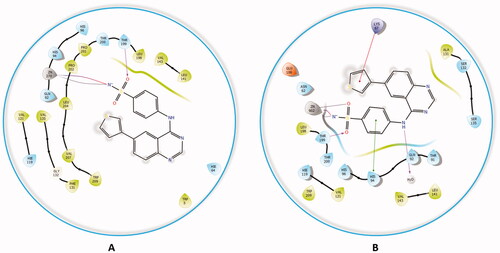
Figure 8. The docked complex of compound 4a with hCA II isoform. (A) 3D model of the crystal structure of hCA II with compound 4a, (B) 3D docking pose of compound 4a, favourable interactions are exhibited as dashed lines: orange‒hydrogen bonds, blue‒π-π stacking, (C) 2D interaction pattern of compound 4a with hCA II binding cavity, favourable interactions are colour coded as follows: green‒π-π stacking, purple‒hydrogen bonds, grey‒metal interactions and light green‒weak van der Waals interactions.