Figures & data
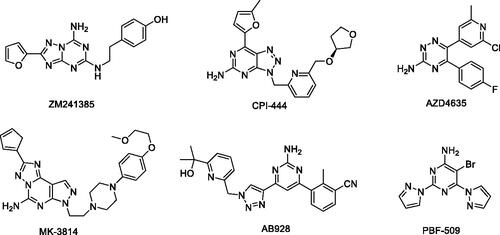
Figure 2. Design of dual A2A/A2B AR antagonists containing quinoline or its open-ring bioisosteres based on the structure of triazole-pyrimidine-methylbenzonitrile.
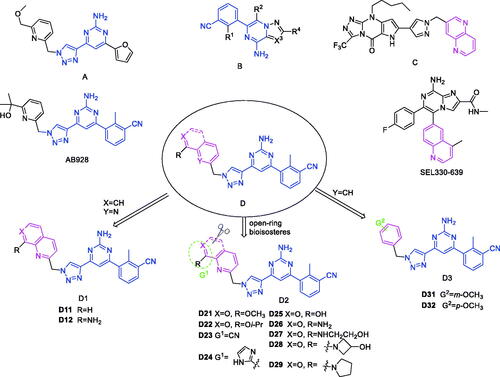
Scheme 1. Synthetic route of target compounds 7a–7n. Reagents and conditions: (a) Pd(dppf)Cl2, (Bpin)2, potassium acetate, 1,4-dioxane, N2, reflux, 4 h; (b) 4,6-dichloropyrimidin-2-amine, K2CO3, Pd(PPh3)4, DMF, N2, 45 °C, 5 min, then compound 2 was added, 115 °C, 5 h; (c) Et3N, TMSA, Pd(PPh3)2Cl2, CuI, dry THF, N2, reflux, 16 h; (d) TBAF(1M in THF), THF, 0 °C to r.t., 20 h; (e) Compound 6, CuSO4·5H2O, sodium l-ascorbate, 60 °C, DMF, t-BuOH, 12 h; (f) SnCl2, 70 °C, 2 h; (g) for 7 h: LiOH, H2O, t-BuOH, r.t., 8 h; for 7i–7l: The corresponding amine derivatives, MeOH, THF, 45 °C, 18 h; (h) TMSiA, DIPEA, THF, r.t., 24 h; (i) DPPA, DBU, THF, r.t., 10 h.
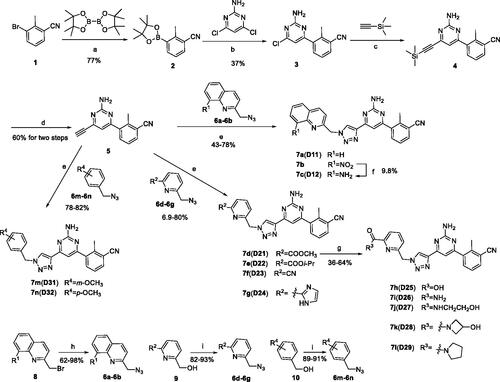
Scheme 2. Synthetic route of the target compound 17. Reagents and conditions: (a) 4,6-dichloropyrimidin-2-amine, K2CO3, Pd(PPh3)4, DMF, N2, 45 °C, 5 min, then compound 11 was added, 115 °C, 5 h; (b) Et3N, TMSA, Pd(PPh3)2Cl2, CuI, dry THF, N2, reflux, 16 h; (c) TBAF(1M in THF), THF, 0 °C to r.t., 20 h; (d) MeMgBr, THF, −10 °C to r.t., 12 h; (e) DPPA, DBU, THF, r.t., 10 h; (f) CuSO4·5H2O, sodium l-ascorbate, 60 °C, DMF, t-BuOH, 12 h.
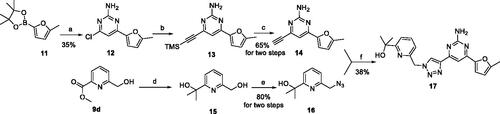
Scheme 3. Synthetic route of the target compound 22. Reagents and conditions: (a) MsCl, Et3N, 0 °C, DCM, 1 h, then TBAF (1 M in THF) was added, 50 °C, 12 h; (b) 4,6-dichloropyrimidin-2-amine, TMSA, Et3N, Pd(PPh3)2Cl2, CuI, dry THF, N2, 80 °C, 12 h; (c) Pd(dppf)Cl2, K2CO3, 1,4-dioxane, N2, 100 °C, 4 h; (d) TBAF (1 M in THF), CuSO4·5H2O, sodium l-ascorbate, H2O, t-BuOH, 60 °C,12 h.
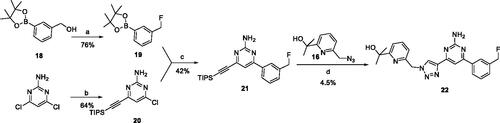
Table 1. Antagonist activity (%inhibition) of compounds 7, 17, and 22 at 10–1000 nM on cAMP levels in CHO cells expressing hA2A AR or hA2B AR a.
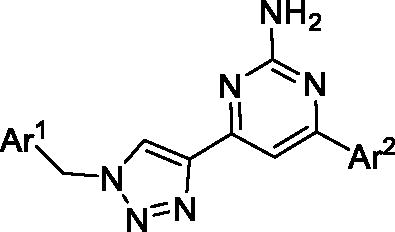
Table 2. IC50 values of selected compounds on cAMP assays in CHO cells expressing hA2A or hA2B ARa.
Table 3. Radioligand binding affinity data (IC50) for selected compounds against hA2A ARa.
Figure 3. The efficiencies of selected compounds for IL-2 production. The efficiencies of selected antagonists on T-cell activation at 10 nM, 100 nM, and 1000 nM were tested in the presence or absence of 1000 nM NECA. The DMSO control was tested in the absence of both antagonists and NECA. The NECA control was tested without antagonists in the presence of 1000 nM NECA.
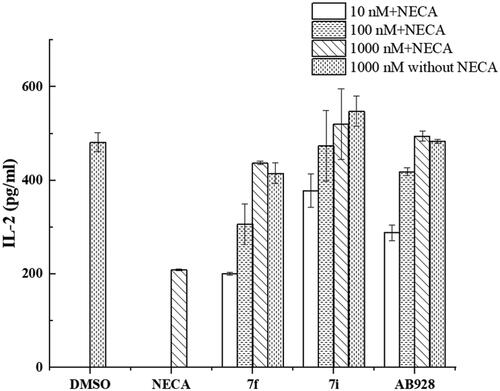
Figure 4. Binding modes of compound 7i at A2A AR (a) and hA2B AR (b) binding cavities have indicated docking orientations and some key receptor residues. Depths of the two binding pockets have been circled with the red dotted line. 2D diagrams of ligand-target interactions for docking compound 7i with the A2A AR (c) and hA2B AR (d) predicted by Discovery Studio.
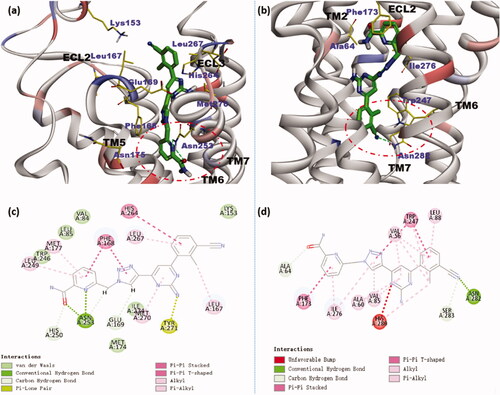
Table 4. Metabolic stability assay in rat and human liver microsomesa.
Figure 5. Plasma concentration-time profiles of compounds 7f and 7i following oral administration (a) and intravenous administration (b).
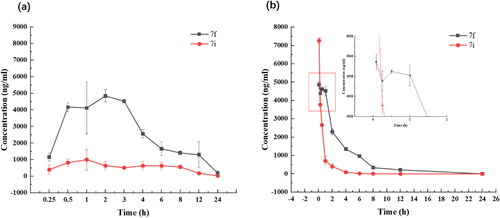
Table 5. Pharmacokinetic parameters of compounds 7f and 7i a.