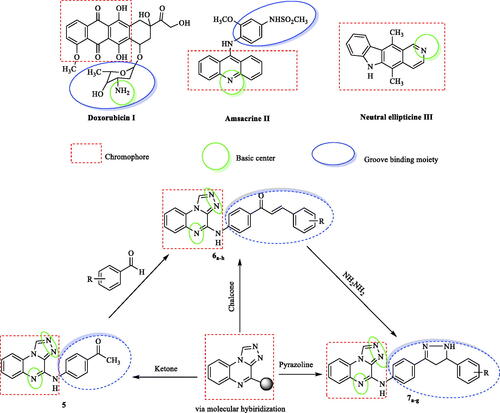
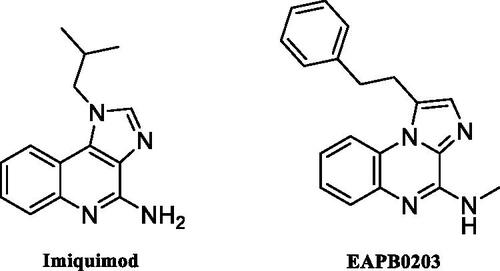
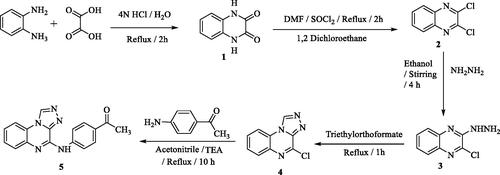
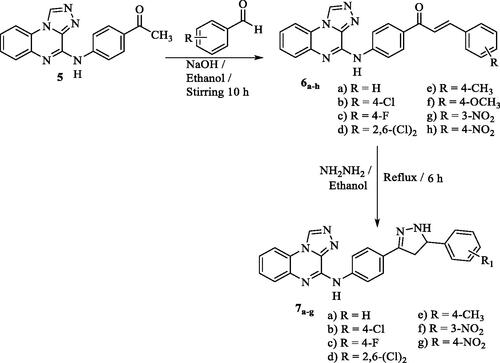
Figures & data
Table 1. Ligands binding affinity (ΔG in Kcal/mole).
Table 2. New derivatives in vitro cell growth inhibitory action.
Table 3. The most potent derivatives; Topoisomerase II inhibitory activity and DNA binding affinity.
Table 4. In silico ADMET calculations.