Figures & data
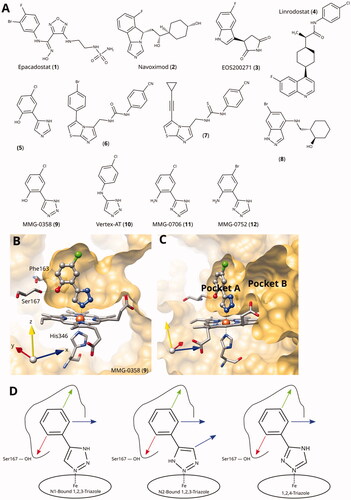
Figure 1. (A) Examples of clinical and other potent IDO1 inhibitors. (B) and (C) Rotated views of the binding pockets A and B in IDO1-active site (PDB ID 6r63Citation22; ligand MMG-0358). (D) Main lead optimisation strategies pursued in this work. The red arrow denotes a preferentially hydrogen-bonding substituent, the green arrow a hydrophobic substituent, and the blue arrows potential access points to pocket B. As demonstrated before, the acidic hydrogens on the triazole rings are crucial for activity and therefore cannot provide access to pocket B.
Scheme 1. General synthesis of 4-aryl-1,2,3-triazoles. Reagents and conditions: (a) TMSA, PdCl2(PPh3)2, Et3N, CuI, dioxane, 45 °C, 5 h. (b) KF, MeOH, rt, 3 h, overall yield 48-85% for 2 steps. (c) TMSN3, CuI, DMF:MeOH (9:1), 100 °C, 10–12 h, yield 44–85%.
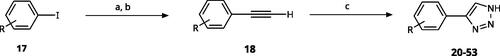
Scheme 2. Synthesis of aryl iodides. Reagents and conditions: (a) aq. NH3, KI, I2, DMF, rt, 1 h, yield 50%. (b) K2CO3, DMF, 80 °C, overnight, yield 50–95%. (c) Cs2CO3, DMF, 100 °C, 24 h, yield 60%. (d) PhCH2P(Br)(Ph)3, [(CH3)3Si]2NNa, THF, rt. (e) N2H4·H2O, FeCl3·6H2O, EtOH, 100 °C, 24 h, overall yield 90% for 2 steps.
![Scheme 2. Synthesis of aryl iodides. Reagents and conditions: (a) aq. NH3, KI, I2, DMF, rt, 1 h, yield 50%. (b) K2CO3, DMF, 80 °C, overnight, yield 50–95%. (c) Cs2CO3, DMF, 100 °C, 24 h, yield 60%. (d) PhCH2P(Br)(Ph)3, [(CH3)3Si]2NNa, THF, rt. (e) N2H4·H2O, FeCl3·6H2O, EtOH, 100 °C, 24 h, overall yield 90% for 2 steps.](/cms/asset/a94a1a13-e6da-4da5-848a-ec4d96166d21/ienz_a_2089665_sch0002_b.jpg)
Table 1. 4-Aryl-1,2,3-triazoles with substituted phenyl groups. 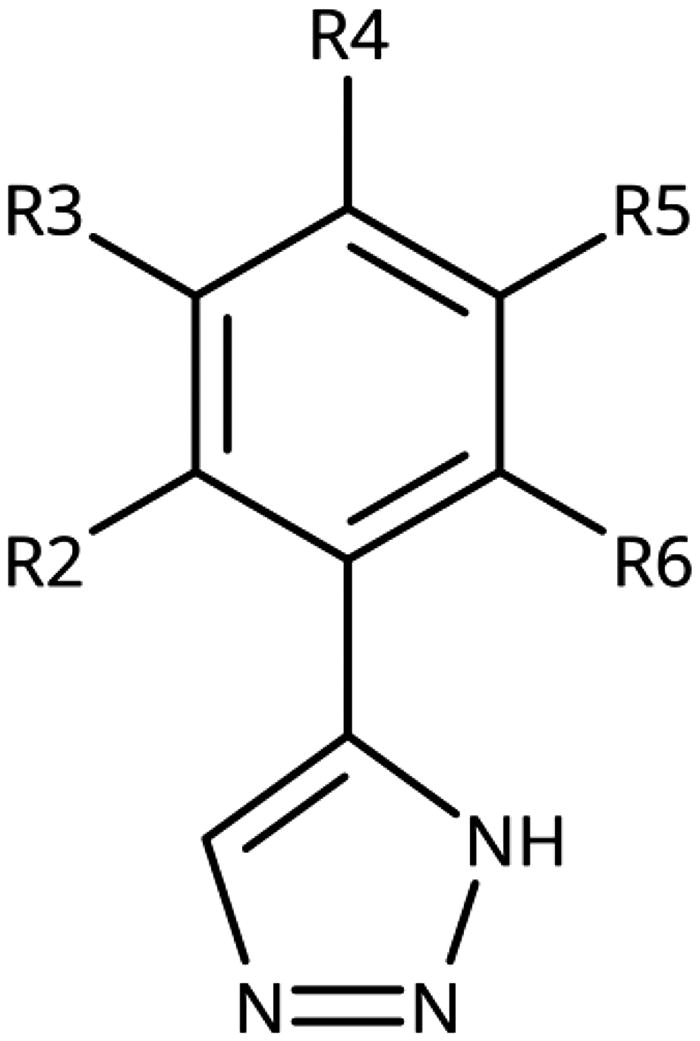
Scheme 3. Synthesis of 5-substituted 4-aryl-(1H)-1,2,3-triazoles. Reagents and conditions: (a) NaN3, DMF, 90 °C, 3 h, yield 75%. (b) Nitroethane, NH4OAc, AcOH, reflux, 2 h. (c) NaN3, pTsOH, DMF, 60 °C, 14 h, overall yield 65–70% for 2 steps. (d) EtOH, rt, 2 h (e) Cs2CO3, DMF, 100 °C, 4 h, overall yield 70% for 2 steps. (f) NaN3, DMSO, 60 °C, 6 h, yield 60%. (g) NaN3, Et3N·HCl, DMF, 70 °C, 10 h, yield 55–65%.
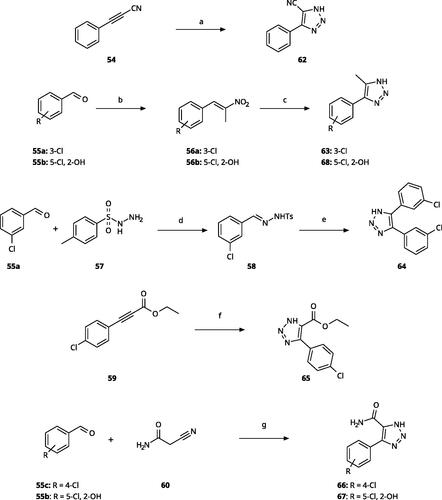
Table 2. 5-Substituted 4-Aryl-1,2,3-Triazoles. 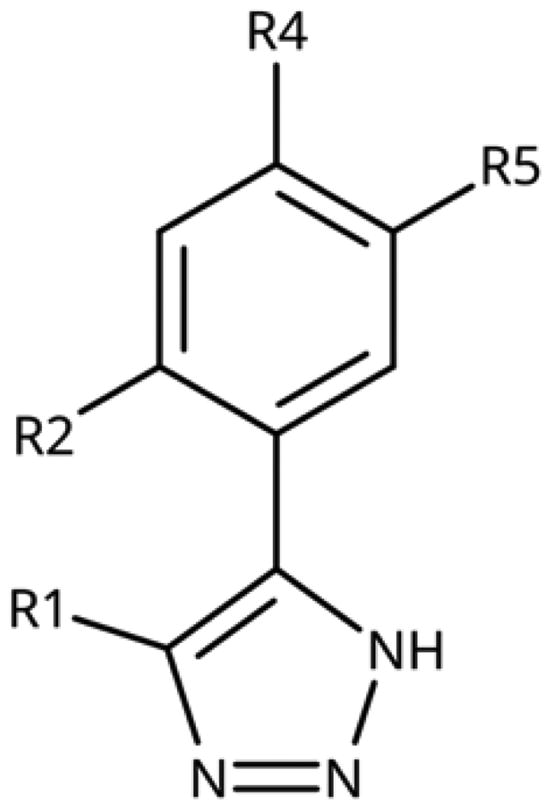
Scheme 4. Aryl-tether-1,2,3-triazoles and annulated derivatives. Reagents and conditions: (a) NaNO2, 2M HCl, CH3COONa, 0 °C-rt, 1 h. (b) EtOH, reflux, overnight, overall yield 70% for 2 steps. (c) TMSN3, CuI, DMF:MeOH (9:1), 100 °C, 10–12 h, yield 50–70%. (d) H2O2, (NH4)2MoO4, MeOH, 0 °C- rt, 14 h, yield 80%. (e) CH3ONH2·HCl, NaOAc, H2O:EtOH, 70 °C, rt, 15 h, yield 55%. (f) N2H4·H2O, KOH, diethylene glycol, 170–190 °C, 6 h, yield 40%.
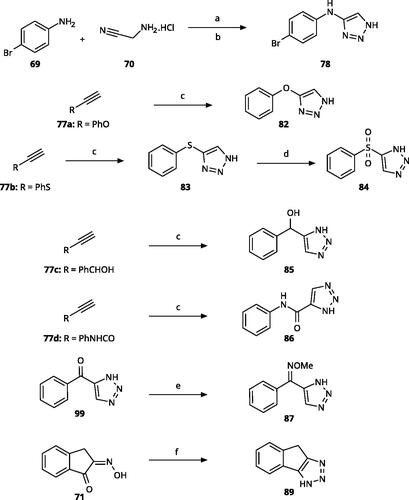
Scheme 5. Synthesis of ethynes. Reagents and conditions: (a) NaOH, DMSO, rt, 4 h. (b) n-BuLi, Et2O, -78 °C-rt, 2h, overall yield 40% for 2 steps. (c) n-BuLi, THF, -78 °C-rt, overnight. (d) KF, MeOH, rt, 4 h, overall yield 85% for 2 steps. (e) propiolic acid, DCC, DMAP, Et2O, rt, 18 h, yield 90%.
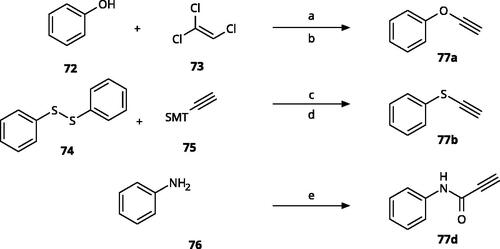
Table 3. 4-Aryl-tether-1,2,3-triazoles and annulated derivatives. 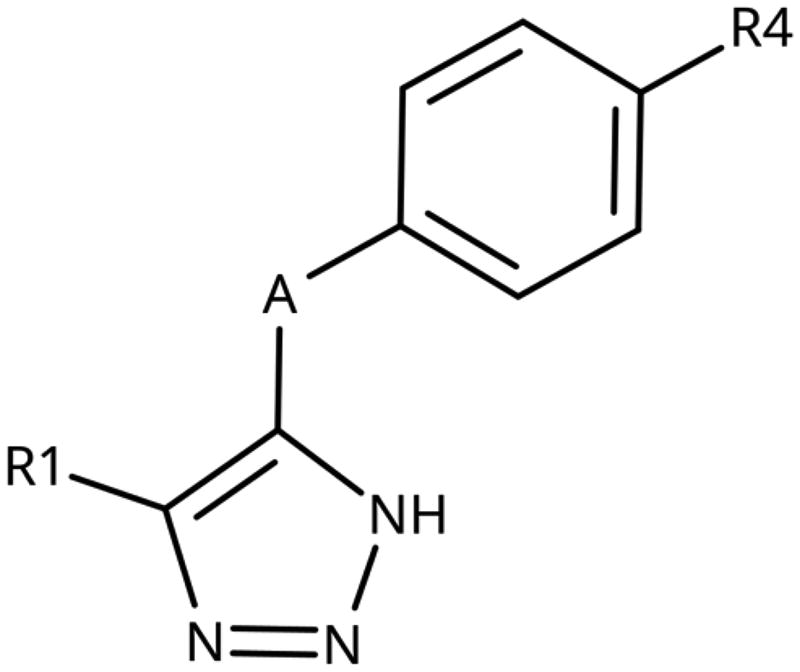
Scheme 6. Synthesis of 5-aroyl and 5-hetaroyl-1,2,3-triazoles. Reagents and conditions: (a) ethynylmagnesium bromide, THF, 3 °C-rt, 1 h. (b) TMSN3, CuI, DMF:MeOH (9:1), 100 °C, 10–12 h, overall yield 55–85% for 2 steps. (c) PCC, DCM, rt, 2 h, yield 35–73%. (d) 48% HBr in H2O, 100 °C, 14 h, yield 45–50%.
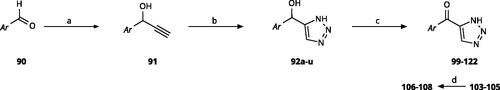
Scheme 7. Synthesis of 4-substituted-5-aroyl-(1H)-1,2,3-triazoles. Reagents and conditions: (a) 4-Bromobenzaldehyde, n-BuLi, -78 °C to -38 °C, THF, 3 h, yield 88%. (b) MnO2, DCM, rt, 5 h, yield 97%. (c) PPTSA, EtOH, 50 °C, 4 h, yield 75%. (d) TMSN3, CuI, DMF/MeOH (9:1), 100 °C, 10–12 h, yield 50%. (e) CBr4, PPh3, DMF, rt, 4 h, yield 85%. (f) 4-Bromobenzoyl chloride, PdCl2(PPh3)2, CuI, Et3N,THF, rt,1 h, yield 90%. (g) TMSN3, CuI, DMF/MeOH (9:1), 10 °C, 10-12 h, yield 45%. (h) TFA, DCM, rt, overnight, yield 75%.
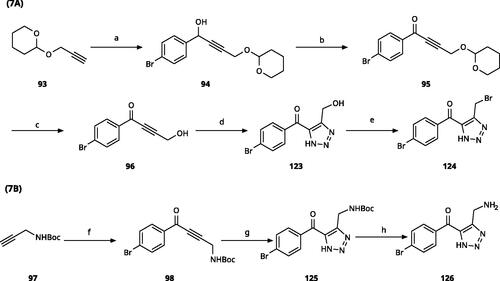
Table 4. 5-Aroyl and 5-Hetaroyl-1,2,3-Triazoles. 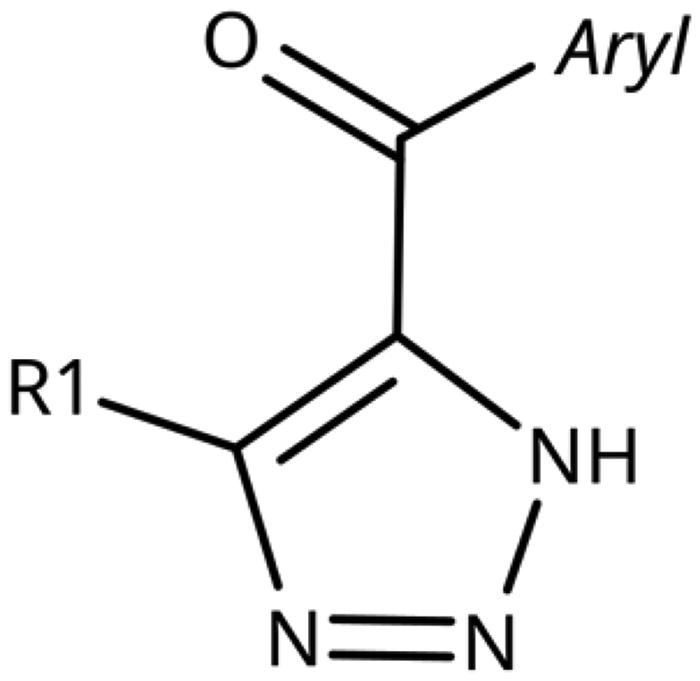
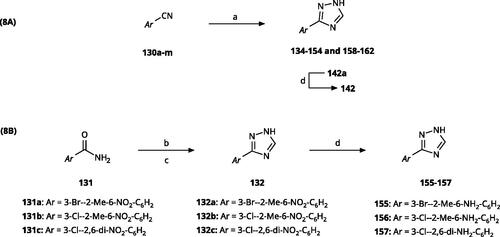
Scheme 8. Synthesis of 3-aryl-1H-1,2,4-triazoles. Reagents and conditions: (a) Formic acid, N2H4·H2O, DMF, 90 °C, 12 h, yield 20–45%. (b) N,N-dimethyl formamide-dimethyl acetal (DMF-DMA), reflux, 1 h. (c) N2H4·H2O, AcOH, 90 °C, 1.5 h, overall yield 70-80% for 2 steps. (d) SnCl2·2H2O, EtOH, 70 °C, 1 h, yield 40–66%.
Scheme 9. Synthesis of aryl carbonitriles and aryl carboxamides. Reagents and conditions: (a) t-BuOK, dimethyl oxalate, N,N-dimethyl acetamide, 140 °C, 5 h, yield 70%. (b) pyridine, DMAP, rt, 3 h, yield 75–85%. (c) HNO3, H2SO4, rt, 3 h (d) (COCl)2, NH4OH, DMF, DCM, reflux, overall yield 70–75% for 2 steps.
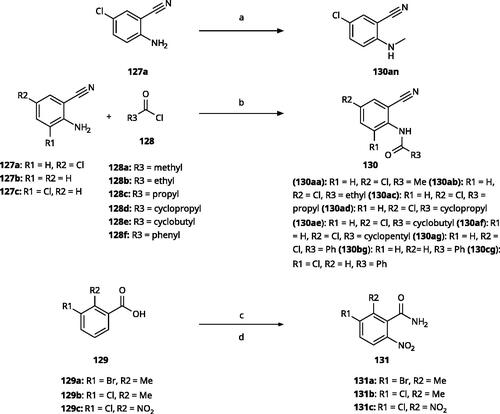
Table 5. 3-Aryl-1,2,4-Triazoles. 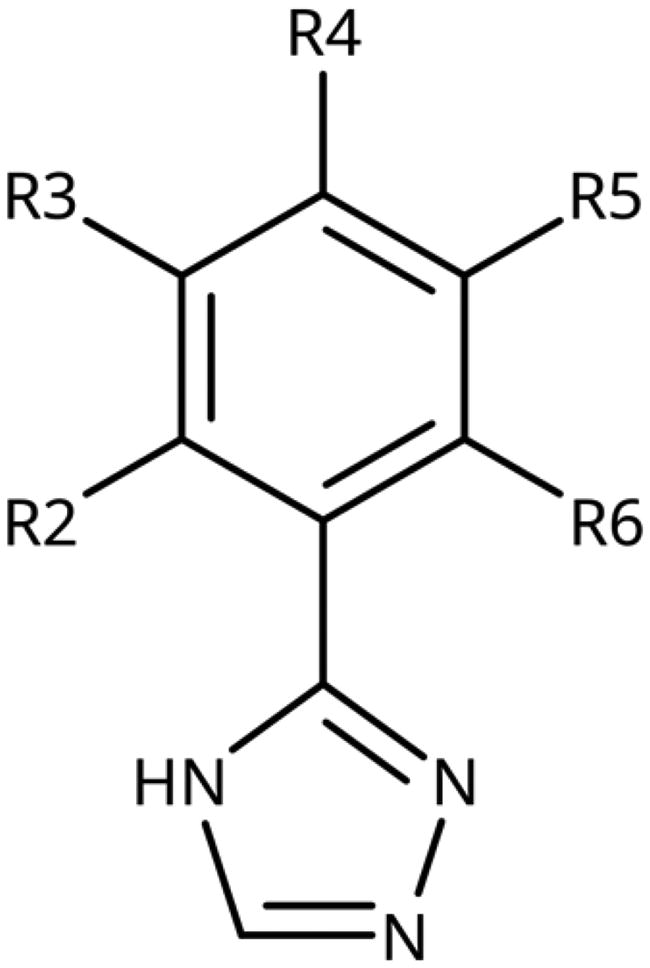
Table 6. 3-Hetaryl-1,2,4-triazoles. 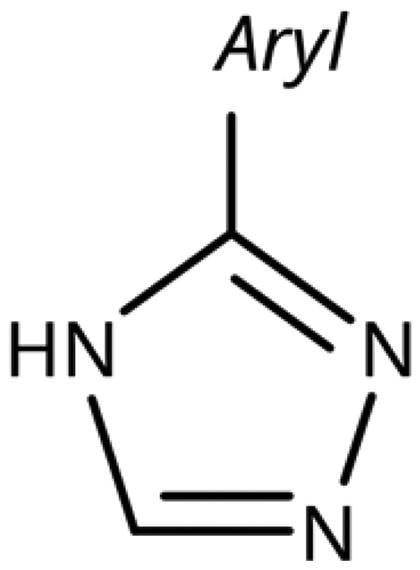
Scheme 10. Synthesis of other 1,2,4-triazoles. Reagents and conditions: (a) K2CO3, CuI, N,N-dimethyl acetamide, 90 °C, 48 h, yield 39%. (b) K2CO3, acetone, reflux, 9h, yield 60%.
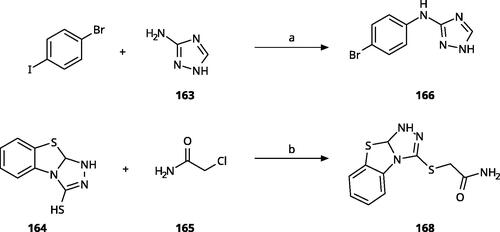
Table 7. Other 1,2,4-triazoles.
Figure 2. (A) Detail of X-ray structure of compound MMG-0472 (13, orange) bound to IDO1 (PDB ID 7zv3). The 2Fo−Fc map of the active site is contoured at 1.0 σ. (B) Same Xray structure, highlighting the His346–iron–ligand bond angle of 170°. (C) DFT-optimized model of MMG-0472 binding to haem. The imidazole–iron–ligand bond angle of 180° is highlighted. (D) Superposition of the X-ray structure of MMG-0472 (orange) and its binding pose predicted by docking (cyan). The RMSD between the structures is 0.3 Å. (E) Top view of MMG-0472 X-ray structure, showing the passage from pocket A to pocket B in-between residues Phe163 on the one side and residues 261–265 (magenta) on the other side. (F) Same view, superimposed with X-ray structures of compounds 9, 11 and 12 (PDB ID 6r63, 7ah5, 7ah6)Citation22,Citation28.
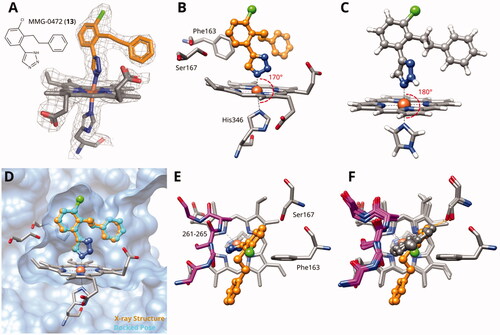
Figure 3. Binding poses of 4-aryl-1,2,3-triazoles predicted by docking. (A) Compound 20: non-optimal pose, the imidazole-iron-triazole bond angle deviates from 180° due to limited space for the p-OH substituent. (B) Compound 22: the m-ethyl substituent is of good size for the hydrophobic subpocket between Val125, Cys129, Leu234 and Gly262. (C) Compound 26: inactive compound with larger ortho substituent using an ether linker. (D) Compound 27: no favourable interactions between hydroxy group and pocket B. (E) Compound 36. (F) Compound 43.
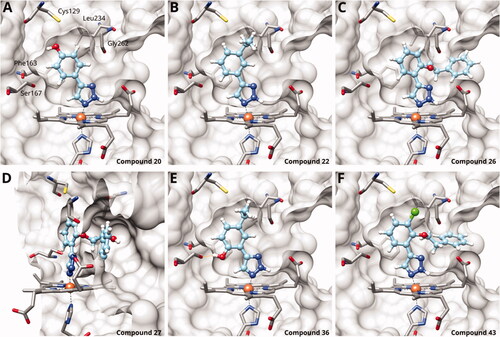
Figure 4. Tethered 1,2,3-triazoles. (A) X-ray structure of compound 10 (PDB ID 6f0a)Citation60. (B) Superposition of X-ray structure and of docking pose of 10. Docking poses of (C) compound 80; (D) compound 83; (E) compound 86; (F) compound 99; (G) compound 102; (H) compound 112; (I) compound 115. Hydrogen bonds are shown in orange.
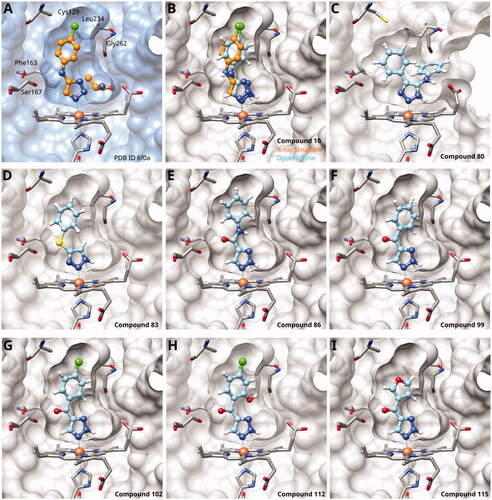
Figure 5. 1,2,4-Triazoles. (A) X-ray structure of compound 11 (PDB ID 7ah5)Citation28. (B) Superposition of X-ray structure and of docking pose of compound 11. Docking poses of (C) compound 141; (D) compound 142; (E) compound 152; (F) compound 155; (G) compound 160; (H) compound 168; (I) compound 171. Hydrogen bonds are displayed in orange.
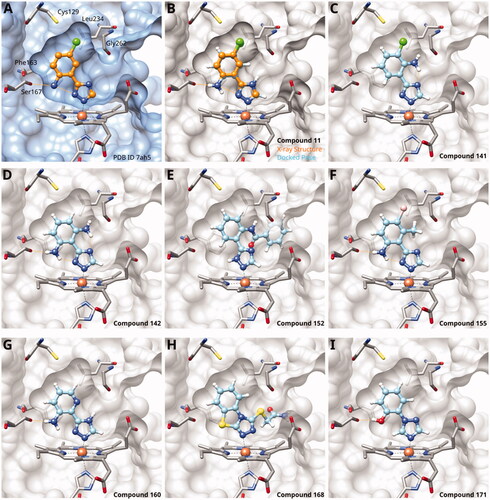
Figure 6. Cellular inhibition and comparison to enzymatic inhibition. (A) Cellular inhibition of kynurenine production at a single compound concentration. Data for 1,2,3-triazole compounds is shown in black, for 1,2,4-triazoles in orange. Values measured at a compound concentration of 200 µM are given in filled bars, values measured at 50 µM in empty bars. (B) Cellular toxicity under the same conditions and using the same colour code as part (A). (C) Cellular inhibition as a function of enzymatic activity (Act = RTlog(IC50)). The dashed line is a sigmoidal fit to all data points except for the marked outliers. Compounds measured at a concentration of 50 µM are marked by empty symbols. (D) Cellular dose-response curves of compounds 140, 143, and 144 measured in this work. (E) Correlation between cellular activity and enzymatic activity for compounds mentioned in this manuscript. Colour code as in part (A). Filled symbols denote the newly determined data shown in part (D).
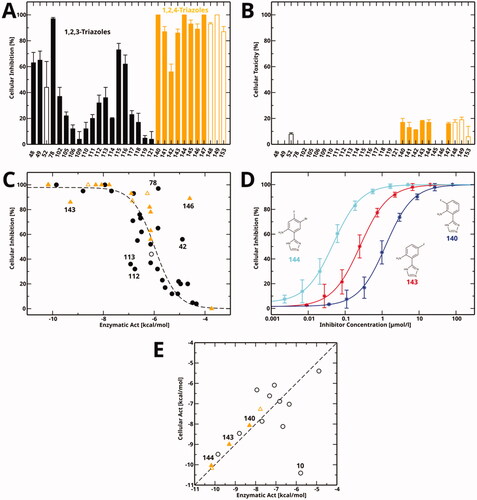
Table 8. Cellular inhibition and toxicity data determined in this work.
