Figures & data
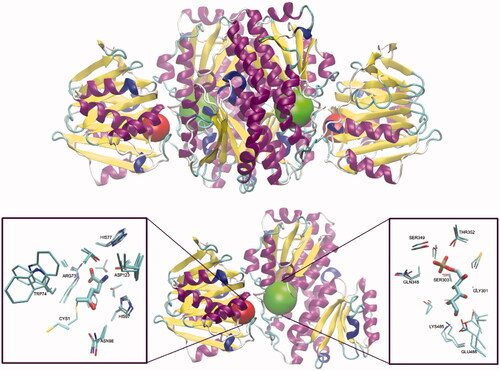
Figure 1. Molecular structure of GlcN-6-P synthase. Top – Structure of E. coli GFA dimer, with GAH and ISOM active centres indicated as red and green spheres, respectively. Based on the pdbid: 1jxa matrix. Bottom – a single subunit of GlcN-6-P synthase, with a detailed presentation of active centres’ crucial residues and 5-oxo-L-norleucine covalently bound to the Cys1 residue at GAH and Glc-6-P in an open ring form at ISOM. Side chains of residues present within the radius of 4.5 Å of both ligands are drawn as thin sticks and ligands as thicker sticks. Crucial residues of superimposed C. albicans (pdbid: 2poc) and H. sapiens (pdbid: 6r4f) GlcN-6-P synthases are shown, to visualise the cross-species conservation of both the structure and conformation of the crucial amino acid residues. The observed significant variations of Cys1, Trp74 and Glu488 confirmations are due to their conformational mobility during the catalytic act.
Scheme 2. Mechanism of sugar phosphate isomerisation by GlcN-6-P synthase. Im and ImH+ represent the non-protonated or protonated form of the imidazole ring, respectively.
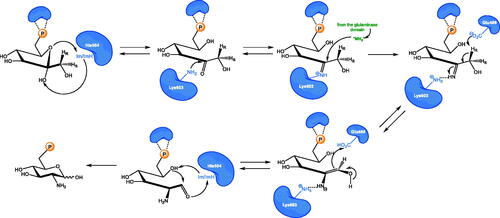
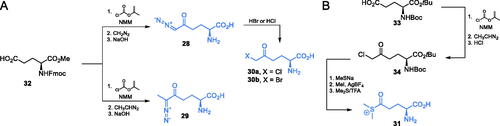
Scheme 3. (A) Antibiotics containing glutamine analogues targeting GlcN-6-P synthase. (B) Synthesis of anticapsin reported by Baldwin et al. Citation36
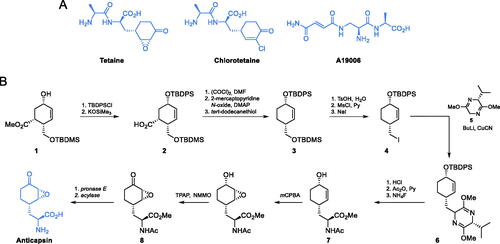
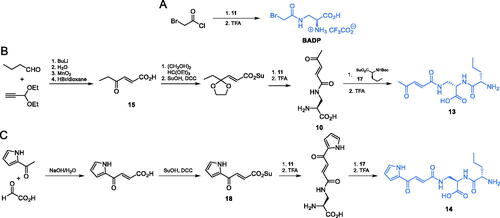
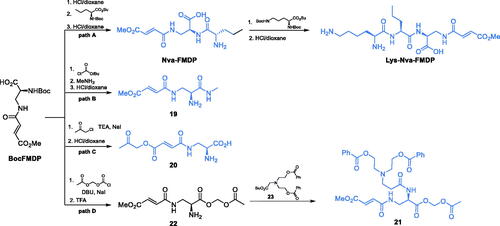
Scheme 4. (A) Synthesis of GlcN-6-P synthase inhibitors containing l-2,3-diaminopropanoic moiety. (B) Molecular mechanism of GlcN-6-P synthase inactivation at GAH by FMDPCitation43.
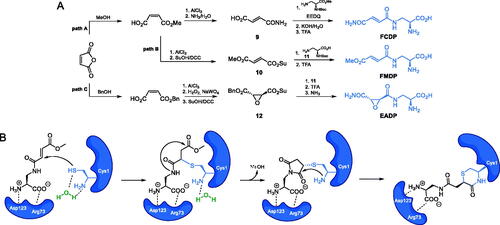
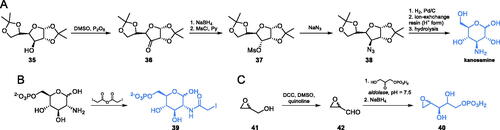
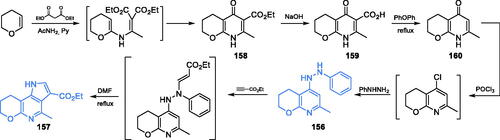
Scheme 7. Synthesis of the mechanism-based GlcN-6-P synthase inhibitor, according to Massiere et al.Citation58
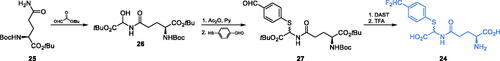
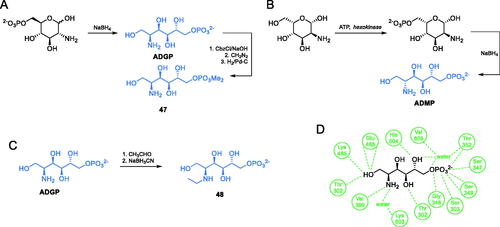
Scheme 11. (A–C) Synthesis of transition state cis-enolamine analogues. (D) ADGP binding at E. coli GlcN-6-P synthase ISOM active site (based on pdbid: 1mos); H-bonds are shown by dashed lines.
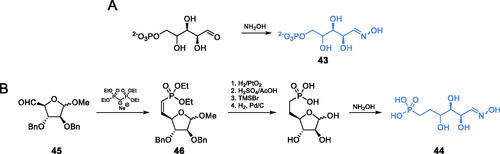
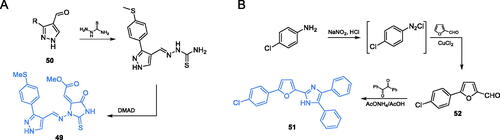
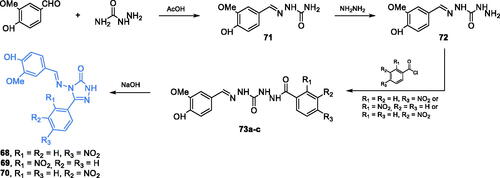
Scheme 12. (A) Syntheses of putative GlcN-6-P synthase inhibitors, according to Vijesh et al.Citation70 (B) Syntheses of presumable GlcN-6-P synthase inhibitors, according to Tomi et al.Citation72
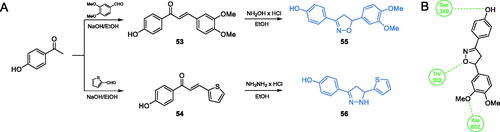
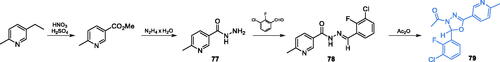
Scheme 13. (A) Synthesis of disubstituted 1,2-oxazole and 1,2-diazole based possible inhibitors of GlcN-6-P synthase, according to Ismail et al.Citation73 (B) Predicted binding mode of compound 55 at the ISOM active site; H-bonds are shown by dashed lines.
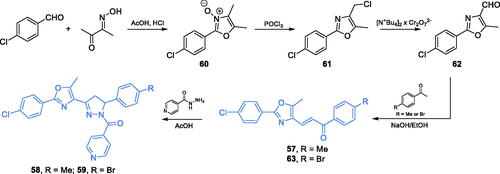
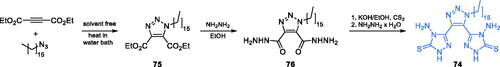
Scheme 14. Synthesis of 1,3-oxazole- and 1,2-diazole-based putative inhibitors of GlcN-6-P synthase, according to Katariya et al.Citation74
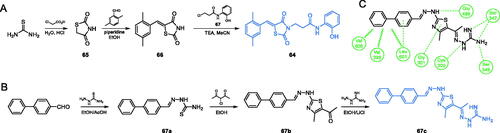
Scheme 15. (A) Synthesis of a potential GlcN-6-P synthase inhibitor, according to Bahare et al.Citation75 (B) Synthesis of GlcN-6-P synthase inhibitor, according to Omar et al.Citation76 (C) Predicted binding mode of 67c at the ISOM active site; H-bonds are shown by dashed lines; hydrophobic interactions are shown by wavy lines.
Scheme 16. Synthesis of triazole-based putative inhibitors of GlcN-6-P synthase, according to Rajasekaran et al.Citation77
Scheme 17. Synthesis of trisubstituted 1,2,3-triazole as a potential inhibitor of GlcN-6-P synthase, according to Aouad et al.Citation78
Scheme 18. Synthesis of 1,3,4-oxadiazoles derivative as a potential inhibitor of GlcN-6-P synthase, according to Shyma et al. Citation79
Scheme 19. Synthesis of 2,5-disubstituted oxadiazole derivative as a potential inhibitor of GlcN-6-P synthase, according to Sindhe et al.Citation82
Scheme 20. Synthesis of 2,4,6-trisubstituted pyridine-based putative inhibitors of GlcN-6-P synthase, according to Kenchappa et al.Citation84
Scheme 21. Synthesis of trisubstituted pyrimidine-based putative inhibitors of GlcN-6-P synthase, according to Venkatesh et al.Citation86
Scheme 22. Synthesis of 2,4,6-trisubstituted 1,3-diazine-based potential inhibitors of GlcN-6-P synthase, according to Bakr et al.Citation87
Scheme 23. Synthesis of barbiturate- and thiobarbiturate-based possible GlcN-6-P synthase inhibitors, according to Kenchappa et al.Citation88,Citation90
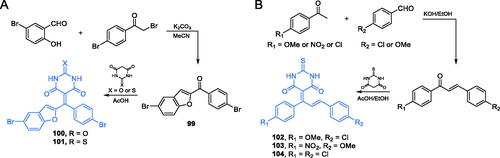
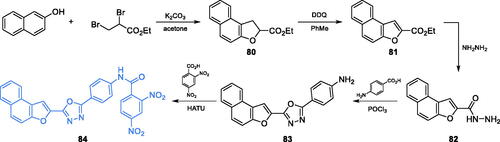
Scheme 24. (A) Synthesis of benzofuran-2-yl derivative-based potential inhibitor of GlcN-6-P synthase, according to Aswathanarayanappa et al.Citation91 (B) Synthesis of β-amino carbonyl derivatives of benzofuran as potential GlcN-6-P synthase inhibitors, according to Kenchappa et al.Citation92
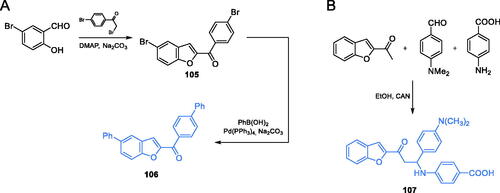
Scheme 25. Synthesis of benzodiazepine-based potential inhibitors of GlcN-6-P synthase, according to Kenchappa et al.Citation93
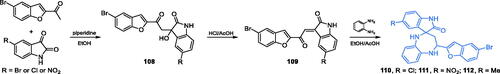
Scheme 26. Synthesis of imidazo[4,5-C]pyridine-based potential GlcN-6-P synthase inhibitor, according to Jose et al.Citation94 and its predicted binding mode to the GAH active centre of GlcN-6-P synthase; H-bonds are shown by dashed lines.
![Scheme 26. Synthesis of imidazo[4,5-C]pyridine-based potential GlcN-6-P synthase inhibitor, according to Jose et al.Citation94 and its predicted binding mode to the GAH active centre of GlcN-6-P synthase; H-bonds are shown by dashed lines.](/cms/asset/5f4fda1a-d3d1-4747-93eb-1446acb8c1bf/ienz_a_2096018_sch0026_c.jpg)
Scheme 27. (A) Synthesis of curcumin derivatives as potential inhibitors of GlcN-6-P synthase, according to Kumar et al.Citation95 (B) Predicted binding mode of compound 117 to the GAH domain of GlcN-6-P synthase; H-bonds are shown by dashed lines.
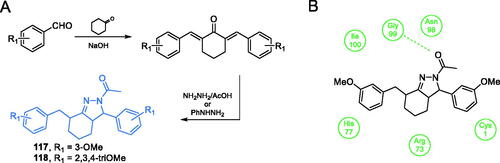
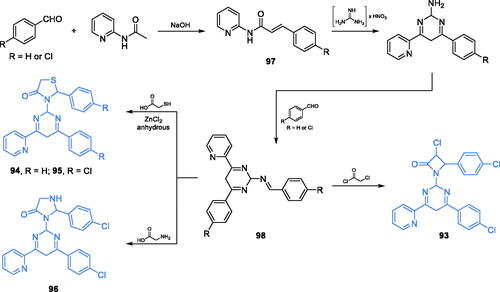
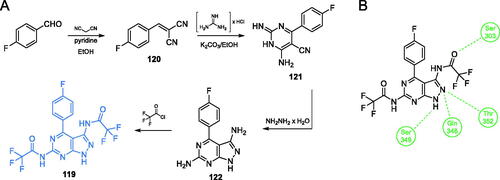
Scheme 28. (A) Synthesis of fluorine-substituted pyrazolopyrimidine-based putative inhibitor of GlcN-6-P synthase, according to Khan et al.Citation97 (B) Presumable binding mode of 119 to GlcN-6-P synthase at the ISOM active site; H-bonds are shown by dashed lines.
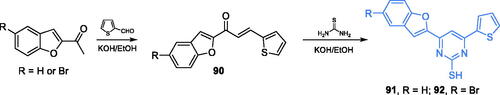
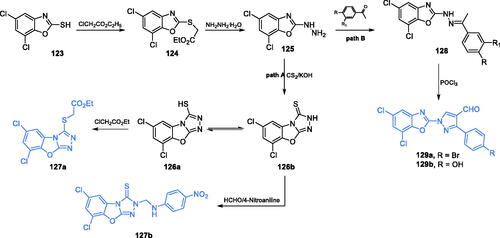
Scheme 29. Synthesis of 5,7-dichloro-1,3-benzoxazole-2-thiol derivatives as potential inhibitors of GlcN-6-P synthase, according to Satyendra et al. (path A)Citation98,Citation99. Synthesis of 5,7-dichloro-1,3-benzoxazole-2-yl derivatives as potential inhibitors of GlcN-6-P synthase according to Jayanna et al. (path B)Citation100.
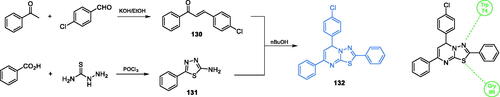
Scheme 30. Synthesis of a potential GlcN-6-P synthase inhibitor, according to Venkatesh et al.Citation101,Citation102 and its predicted binding mode to the GAH domain; H-bonds are shown by dashed lines.
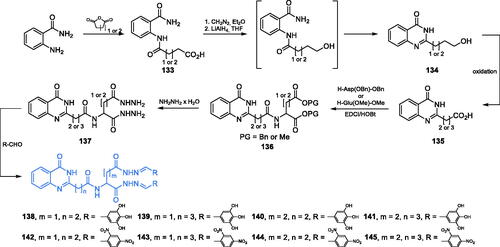
Scheme 31. Synthesis of quinazolinone-based putative inhibitors of GlcN-6-P synthase, according to Kumara et al.Citation103
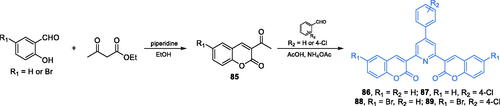
Scheme 32. (A) Syntheses of coumarin based potential inhibitors of GlcN-6-P synthase, according to Kenchappa (path A)Citation92, Kumar (path B)Citation107 and Helmy et al. (path C)Citation108. (B) Synthesis of 4-chromone-based inhibitor of GlcN-6-P synthase, according to Devi et al.Citation109
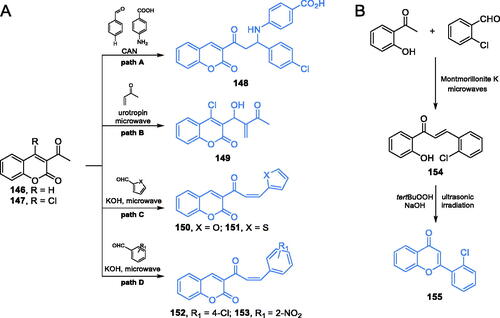
Scheme 33. Synthesis of potential GlcN-6-P synthase inhibitors, according to Elkanzi et al.Citation110
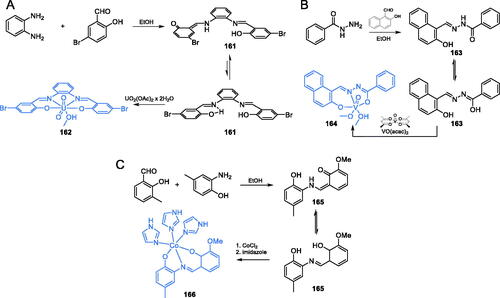
Scheme 34. Syntheses of: (A) uranyl(VI) complex; (B) vanadium(V) complex; (C) cobalt(II) complex with antimicrobial activity, according to Ebrahimipour et al.Citation113–115
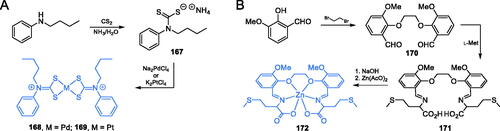
Scheme 35. Synthesis of metal complexes, potential GlcN-6-P synthase inhibitors, according to (A) Onwudiwe et al.Citation116 and (B) Wang et al.Citation118
Scheme 36. Structure of aaptamine and synthesis of RO0509347 developed at Hoffman La-Roche Inc.Citation122
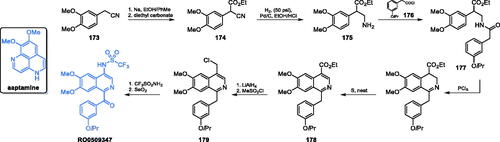
Figure 2. The hypothetical complex of RO0509347 with human GlcN-6-P synthase. Drawing based on the results of docking calculations to the hGFAT2 matrix (pdbid: 6r4f)Citation123, performed with the use of Autodock 4.2, according to the procedure described previouslyCitation124. A single subunit of the tetrameric enzyme is shown, with GAH and ISOM active centres indicated as red and green spheres respectively. A flexible linker joining both domains of the enzyme is coloured blue.
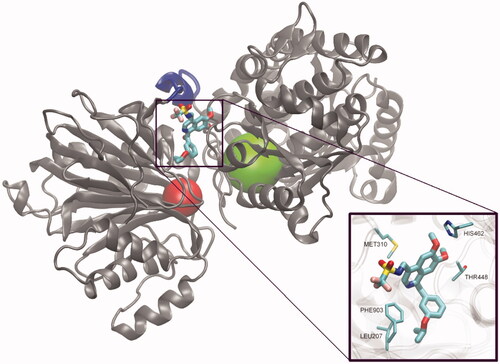
Scheme 37. (A) Synthesis of 1,2-diazole-based inhibitors of GlcN-6-P synthase according to Khan et al.Citation125 (B) Synthesis of trisubstituted 1,2-diazole-based inhibitors of GlcN-6-P synthase according to Ebenezer et al.Citation126
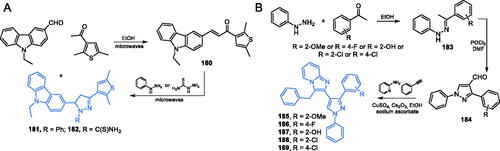
Scheme 38. (A) Syntheses of a possible GlcN-6-P inhibitor, according to Sarojini et al.Citation127,Citation128 and its predicted binding mode to GlcN-6-P synthase; H-bonds are shown by dashed lines.
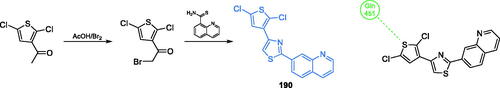
Scheme 39. Synthesis of trisubstituted 1,2,4-triazole as a potential inhibitor of GlcN-6-P synthase, according to Krishna et al.Citation129
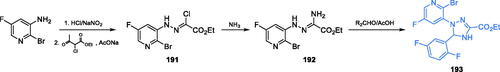
Scheme 40. Synthesis of the 1,3,4-thiadiazole derivative as a putative inhibitor of GlcN-6-P synthase, according to Siwek et al.Citation130
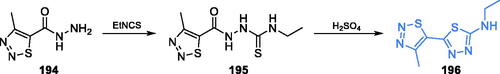
Scheme 41. Synthesis of spiro pyrrolidine-based putative inhibitor of GlcN-6-P synthase, according to Askri et al.Citation132
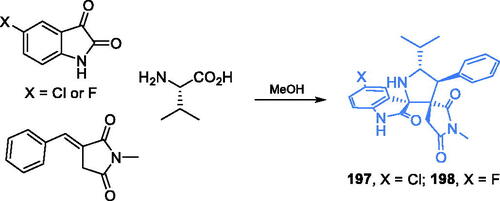
Scheme 42. (A) Synthesis of fluorinated pyridazinone derivatives as a potential inhibitor of GlcN-6-P synthase, according to Sowmya et al.Citation133 (B) Synthesis of pyridazinone derivatives as a putative inhibitor of GlcN-6-P synthase, according to Nagle et al.Citation134
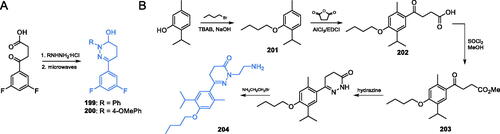
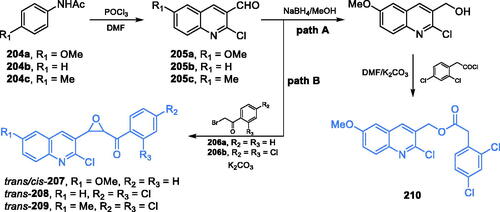
Scheme 43. Synthesis of 2-chloroquinolin-3-yl ester derivatives as potential GlcN-6-P inhibitors, according to Tabassum et al. (path A)Citation139. Synthesis of quinoline-based epoxides, according to Preveena et al. (path B)Citation138.
Scheme 44. Synthesis of isoquinoline derivatives as potential inhibitors of GlcN-6-P synthase, according to Borse et al.Citation140