Figures & data
Table 1. In vitro anti-proliferative effects of the obtained compounds against HepG2 and MCF-7 cell lines. 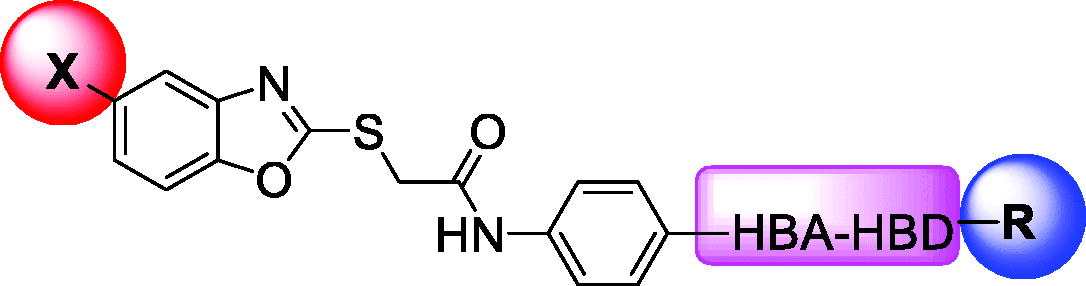
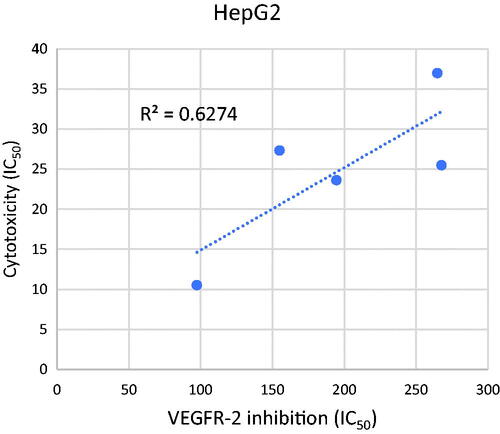
Table 2. IC50 values of the tested compounds on the inhibitory activities against VEGFR-2 Kinases Assay.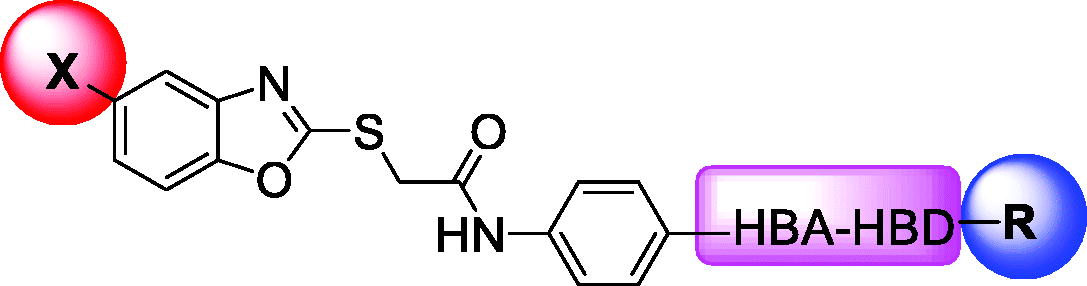
Table 3. IC50 results of 12d, 12i, and 12 l against WI-38 cell line.
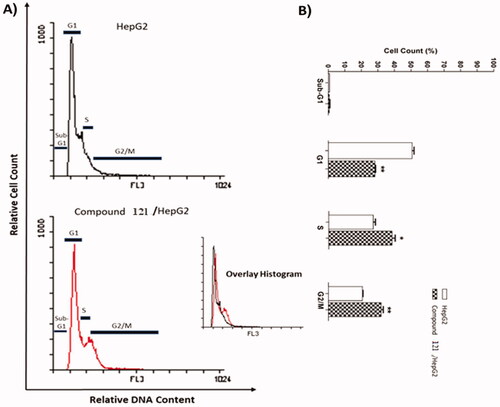
Table 4. Supressing potentialities of 12I on the cell cycle of HepG2 cells after 24 h treatment.
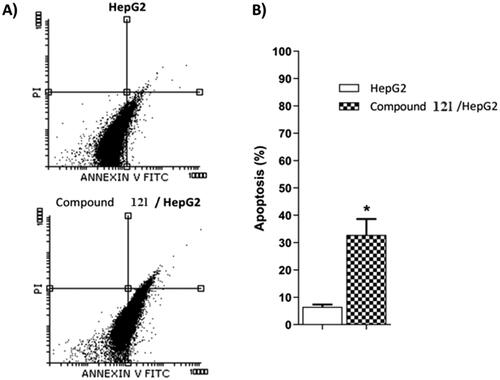
Table 5. Apoptotic potentialities compound 12 l against HepG2 cells after 24 h treatment.
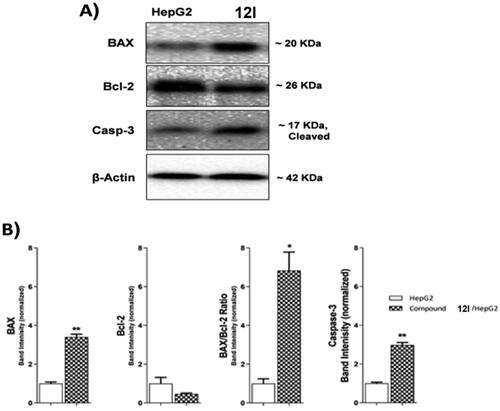
Table 6. Effect of compound 12 l on the levels of BAX, Bcl-2, and Caspase-3 proteins expression in HepG2 cells treated for 24 h.
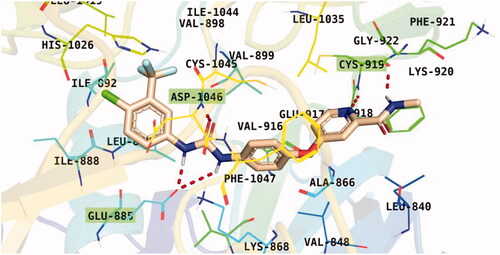
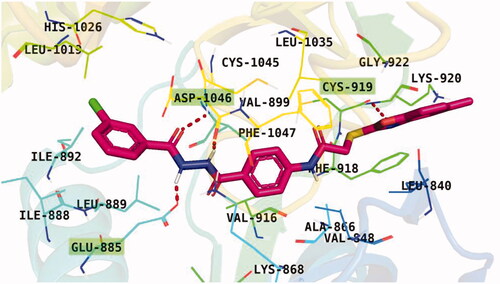
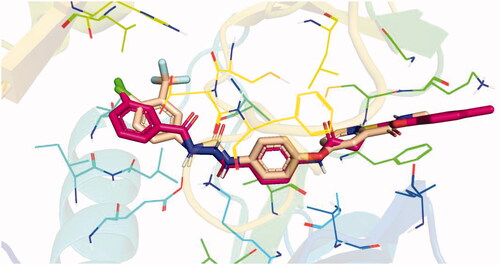
Figure 7. Results of the re-docking step into the VEGFR-2 catalytic site; native ligand (green) and the obtained pose (red).
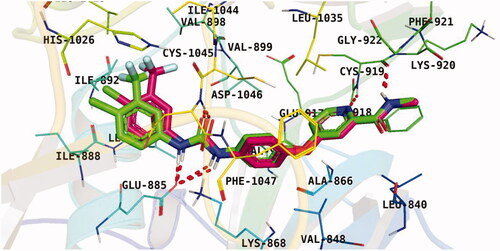
Table 7. Physicochemical properties of the tested compounds passed Lipinski and Veber Rules
Table 8. ADME profile of compounds 12d, 12i, and 12 l
Table 9. Radar charts for prediction of oral bioavailability profile of compounds 12d, 12i, and 12 l
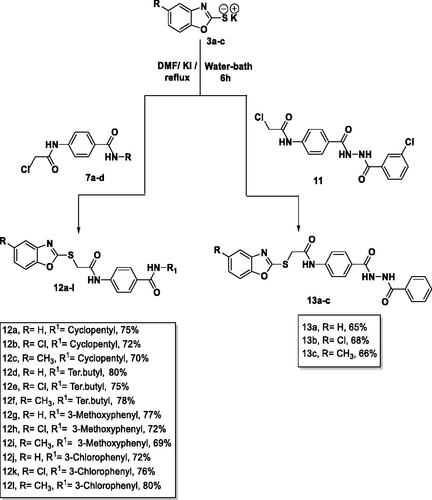
Table 10. Colours, yields, and meting points of the target compounds