Figures & data
Scheme 1. Chemical structures of investigated pyrithionato ligands a–h, synthesis of zinc complexes 1a–h and organoruthenium complex 2a.
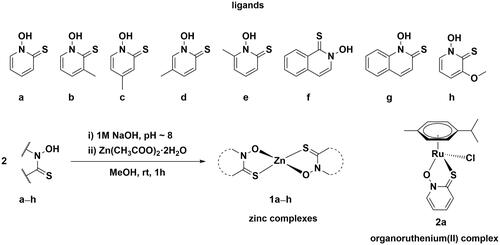
Figure 1. Crystal structure of complexes 1a and 1d (together with zoom of trigonal bipyramidal and tetrahedral coordination, respectively). Ellipsoids are drawn at the 35% probability level. Additional data can be found in SI (Supplementary Figure 2).
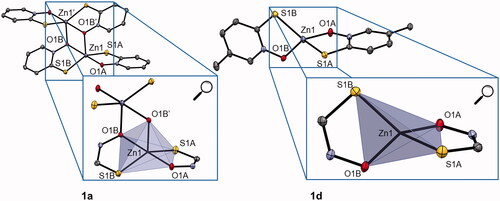
Figure 2. Effect of pyrithione complex with zinc 1a and with ruthenium 2a on cathepsin L and SARS-CoV-2 PLPro. The effect of pyrithione (ligand a) is also shown. Data are presented as mean ± S.E.M. (N = 3).
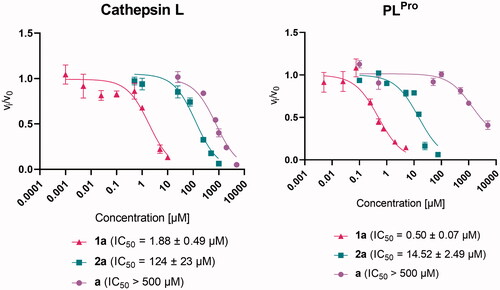
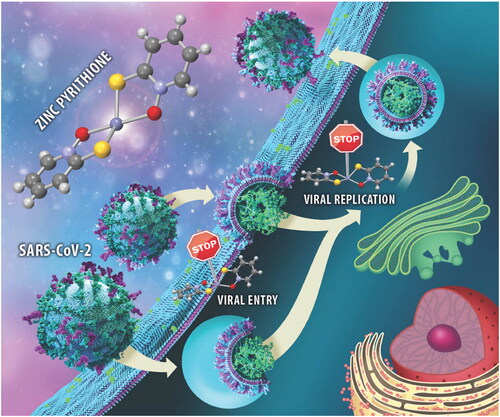
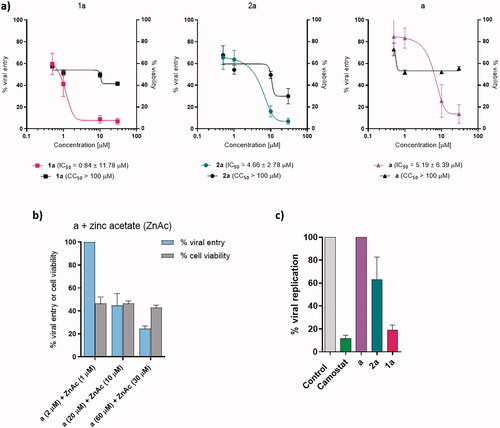
Figure 3. Viral entry, viral replication, and cell survival assays. (a) Viral entry assay of a, 1a, and 2a together with cell viability. Data are presented as mean ± S.E.M. (n = 3 independent experiments with technical triplicates for viral entry and duplicates for cell viability). (b) Viral entry assay after the application of pyrithione a and zinc acetate together with cell viability. Data are presented as mean ± S.E.M. (n = 1 with technical triplicates for viral entry and duplicates for cell viability). (c) Viral replication-competent assay for a, 1a, and 2a. Data are presented as mean ± S.E.M. (n = 1 with technical triplicates). Camostat was used as a positive control of viral inhibition.
Supplemental Material
Download PDF (2 MB)Data availability statement
The additional data that support the findings of this study are available from the corresponding author upon reasonable request. The manuscript together with SI has been uploaded to bioRxiv preprint server with https://doi.org/10.1101/2022.03.03.482819.Citation80
