Figures & data
Figure 2. (A) The docking poses of 5a-e for chloro, bromo, nitro, fluoro and methyl substituents, as cyan, pale rose, orange and purple sticks, respectively, in the binding site of COX-2. (B) Focussed view at the substituents of 5a-e. (C), (D) and (E), Focussed view at substituents isopropyl, 5-membered dummy ring and 6-membered phenyl groups, respectively. Polar and non-polar regions of the binding site were presented by red and green coloured molecular surface, respectively. Dashed lines indicate favourable interactions, while red arrows represent steric clashes and unfavourable interactions. Non-polar hydrogen atoms were omitted for clarity.
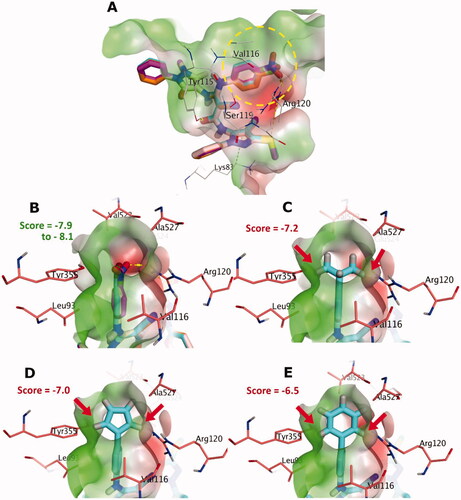
Figure 3. Synthesis of the target compounds 5a-e. Reagents and reaction conditions: i: C6H5NH2, EtOH, Reflux; ii: cyanoacetic acid, acetic anhydride, 50 °C; iii: 4-R-C6H4NCS, KOH, DMF, 0 °C; iv: 4-aminoantipyrine, oil bath at 170 °C.
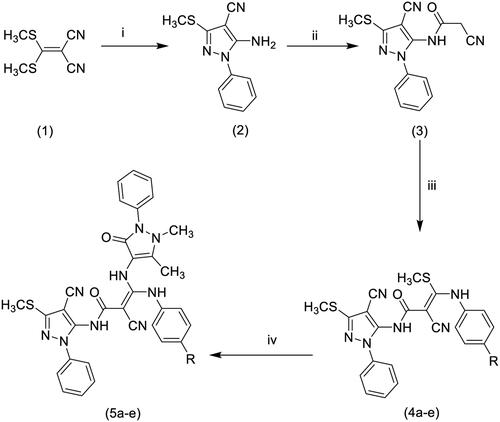
Table 1. The docking score of the active compounds in the binding site of COX-2.
Figure 4. (A) Overlay of the docking pose of 5a (cyan), 5b (pale rose), 5d (orange) and 5e (purple) in the binding site of COX-2 (PDB: 6BL4). Non-polar hydrogen atoms were omitted for clarity. (B) Interactions of 5a docking pose with COX-2 protein in a 2 D depiction.
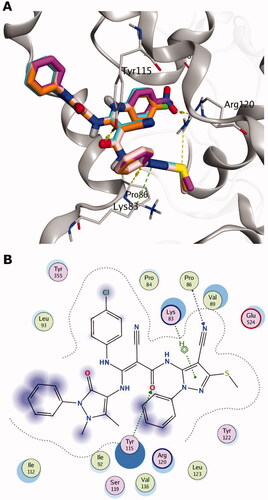
Figure 5. (A) Root mean square deviation (RMSD) of the protein alpha carbon atoms during the simulation for the apo and complexed forms with 5d and 5e. (B) Radius of gyration (Rg) for the COX-2 protein in the apo and complexed forms with 5d and 5e during the simulation time. The 50000 ps simulation time (x- axis) implies 50 ns time. (C) Per residue root mean square fluctuation (RMSF) for the apo and complexed forms with 5d and 5e. (D) and (E) Hydrogen bond count during the MD simulation for both 5d and 5e in the binding site, respectively.
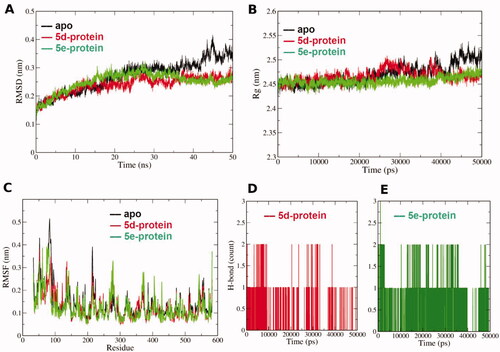
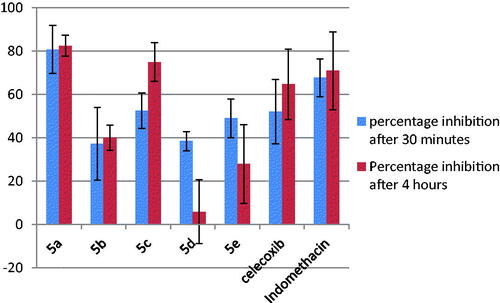
Table 2. COX-1 and COX-2 inhibition of the tested compounds.
Table 3. Real-time PCR results.
Table 4. Acute ulcerogenic side effect of the tested compounds.