Figures & data
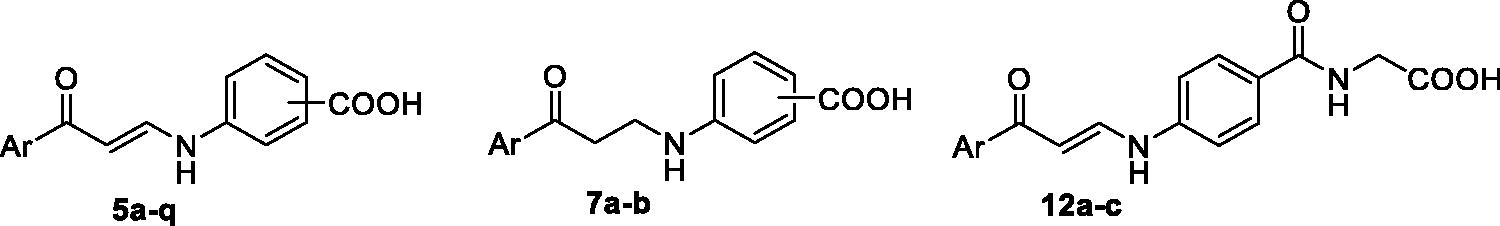
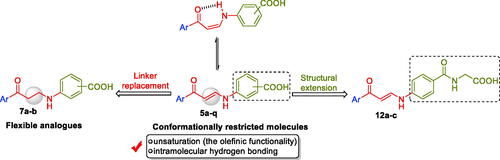
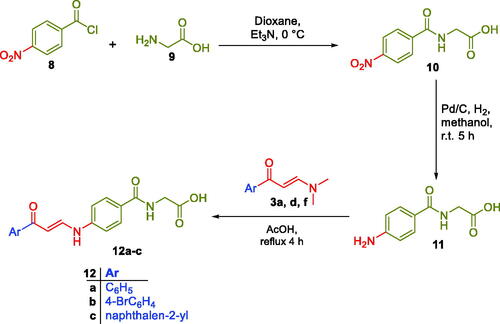
Scheme 1. Synthesis of benzoic acids-bearing enaminones 5a–q and 3/4-((3-oxo-3-phenylpropyl)amino)benzoic acids 7a–b.
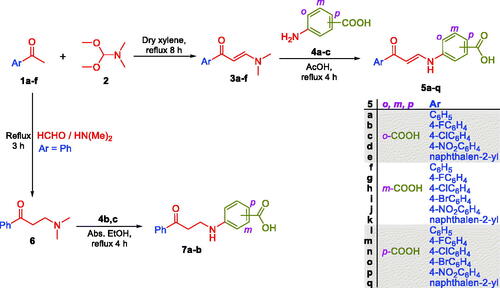
Figure 4. The existence of ortho-substituted carboxylic acids enaminones 5a–e in Z-form and the existence of meta- and para-substituted carboxylic acids enaminones 5f–k and 5l–q in E/Z-forms (1:1) in DMSO.
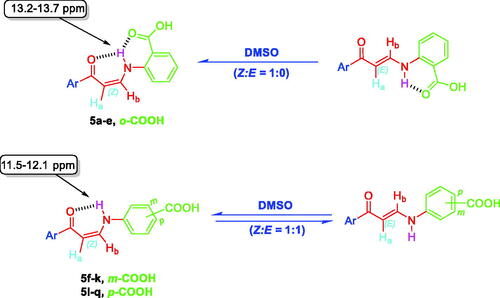
Figure 5. 1H NMR of enaminone 5i showing the presence of Z-form [Z Ha (d), Z Hb (dd), E NH (d)] and E-form [E Ha (d), E Hb (overlapped dd), E NH (d)] in DMSO as a representative example for enaminones 5f–k and 5l–q.
![Figure 5. 1H NMR of enaminone 5i showing the presence of Z-form [Z Ha (d), Z Hb (dd), E NH (d)] and E-form [E Ha (d), E Hb (overlapped dd), E NH (d)] in DMSO as a representative example for enaminones 5f–k and 5l–q.](/cms/asset/734ecf03-37bc-46e2-bc3b-f403d884cd59/ienz_a_2114079_f0005_c.jpg)
Figure 6. The presence of meta- and para-substituted carboxylic enaminones 5f–k and 5l–q in Z/E-forms in DMSO and in D2O/DMSO (1H NMR).
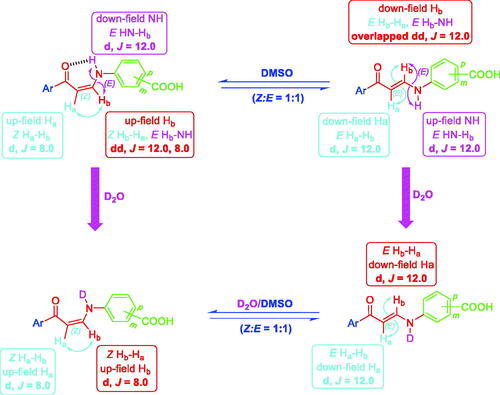
Figure 7. 1H NMR of enaminone 12b which showed the existence of Z- and E-form in DMSO as a representative example for 12a–c.
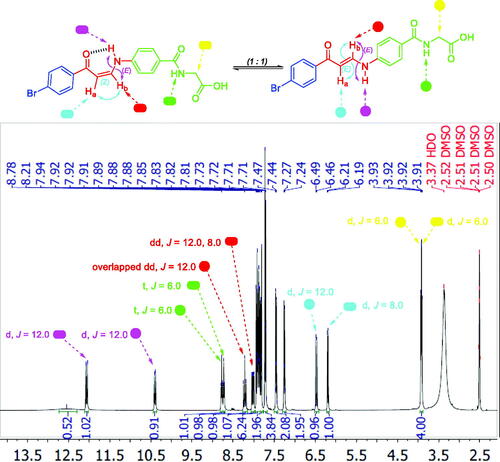
Table 1. hCA I, II, IX and XII inhibition results from the carboxylic acid derivatives 5a–q, 7a–b and 12a–c and acetazolamide (AAZ) as a reference CA inhibitor.