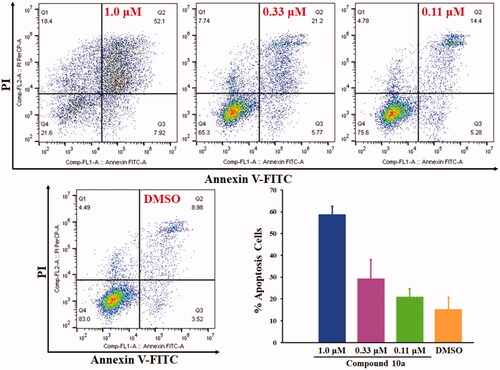
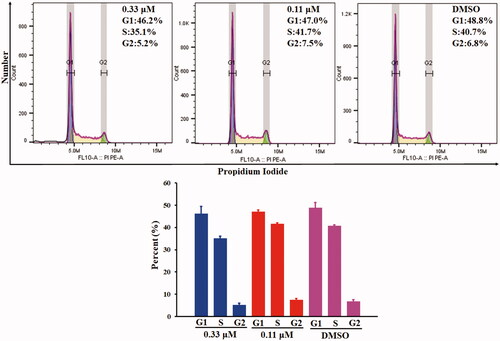
Figures & data
Figure 1. The structures of Regorafenib, Fruquintinib, Foretinib, BMS-777607, MK-8033, Crizotinib, Cabozantinib and BC2021-104511-15i.
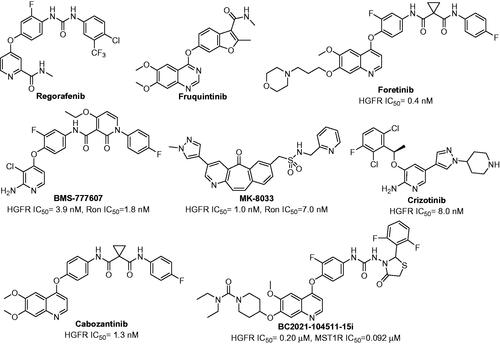
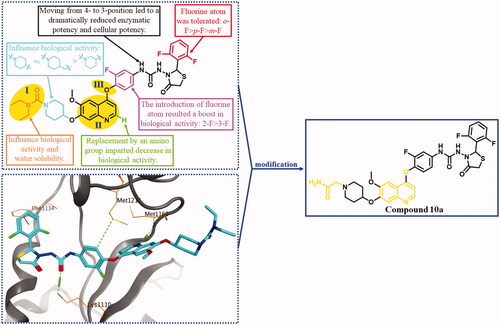
Figure 2. The binding mode of BC2021-104511-15i with HGFR (PBD ID: 3LQ8), the SARs of lead compound BC2021-104511-15i and the modification of fragments I, II and III. Lead compound was shown by blue sticks, and the H-bonds were represented by green dotted lines, and the H-arene interaction was shown by red dotted lines.
Scheme 1. Synthesis of target compounds 10a–i. Reagents and conditions: (i) 1a: 2-fluoro-4-nitrophenol, PhCl, reflux, 14 h; 1b: 2-fluoro-4-nitroaniline, i-PrOH, conc. HCl, reflux, 3 h; (ii) 33% HBr in HOAc, rt, 3 h; (iii) 1-Boc-4-methanesulfonyloxypiperidine, Cs2CO3, DMF, 110 °C, 6 h; (iv) Fe, 90% EtOH-H2O, conc. HCl (cat.), reflux, 4–6 h; (v) phenyl chloroformate, pyridine, CH2Cl2, rt, 2 h; (vi) 50% hydrazine hydrate, xylene, 70 °C, 2 h; (vii) 2, 6-difluorobenzaldehyde, i-PrOH, HOAc (cat.), reflux, 2–3 h; (viii) CF3COOH, CH2Cl2, rt, 2 h; (ix) RCOCl, Et3N, CH2Cl2, rt, 4–5 h; R-Cl, Cs2CO3, DMF, 90 °C, 6–8 h; (x) mercaptoacetic acid, SiCl4, CH2Cl2, reflux, 6–8 h; (xi) MeOH, NaOH, 50 °C, 1 h.
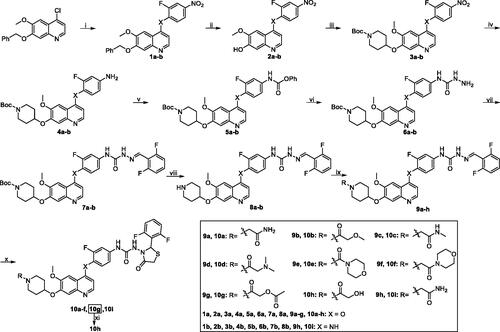
Scheme 2. Synthesis of target compound 19. Reagents and conditions: (i) 2-fluoro-4-nitrophenol, PhCl, reflux, 14 h; (ii) 33% HBr in HOAc, rt, 3 h; (iii) 1-Boc-4-methanesulfonyloxypiperidine, Cs2CO3, DMF, 110 °C, 6 h; (iv) Fe, 90% EtOH-H2O, conc. HCl (cat.), reflux, 4 h; (v) (1) phenyl chloroformate, pyridine, CH2Cl2, rt, 2 h; (2) 50% hydrazine hydrate, xylene, 70 °C, 2 h; (vi) 2, 6-difluorobenzaldehyde, i-PrOH, HOAc (cat.), reflux, 3 h; (vii) CF3COOH, CH2Cl2, rt, 2 h; (viii) 2-chloroacetamide, Cs2CO3, DMF, 90 °C, 8 h; (ix) mercaptoacetic acid, SiCl4, CH2Cl2, reflux, 6 h.
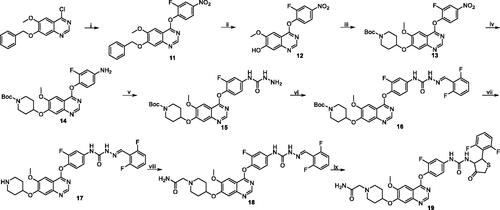
Figure 3. Mimetic binding mode of compound 10a (yellow sticks) within HGFR (PDB ID: 3LQ8). The H-bonds were represented by green dotted line and the H-π was represented by red dotted lines.
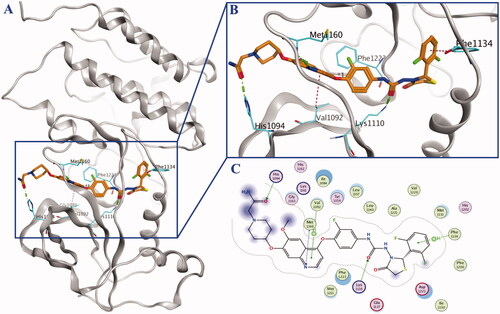
Table 1. The structures of target compounds 10a–i and 19 and their inhibitory activity against HGFR, MST1R, HT-29, HCT-116 and COLO 205 cells.
Table 2. The cytotoxicity of selected compounds against FHC cells.
Table 3. The kinase inhibitory activity of compounds 10a and 10b against ABL, PDGFRβ, AXL, FLT3, RET, c-Src and VEGFR-2.