Figures & data
Figure 1. Role of mitochondrial and cytosolic CA isoforms in fatty acid biosynthesis: the transfer of acetyl groups from the mitochondrion to the cytosol (in the form of citrate) is required for the provision of substrate for de novo lipogenesis via malonyl-coenzyme A as key intermediate. Steps involving bicarbonate, both for the reaction catalysed by pyruvate carboxylase (PC) and acetyl-coenzyme A carboxylase (ACC) require the presence of CA isozymes: CA VA/VB in the mitochondrion and CA II in the cytosol.
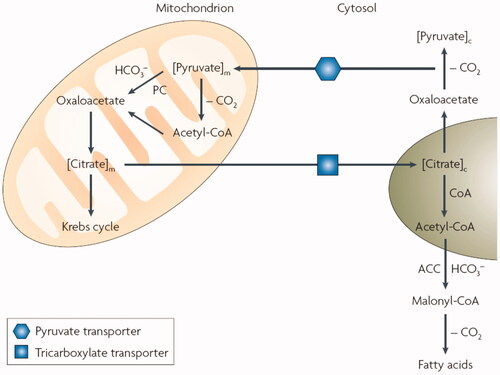
Figure 2. Sulphonamide/sulfamate CAIs in clinical use: acetazolamide (AAZ), zonisamide (ZNS) and topiramate (TPM).
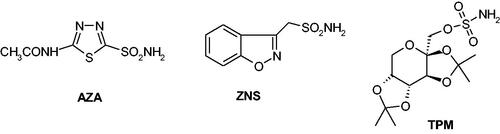
Table 1. CA inhibition data with selected drugs/investigational compounds against human (h) isoforms hCA I, II, VA and VBCitation13,Citation51,Citation52.
Figure 3. (A) hCA II complexed with superimposed topiramate (PDB 3HKU) in green, and zonisamide (PDB not deposited, available from the authorsCitation52) in magenta. The Zn(II) is shown as a grey sphere that is bound to the protein ligands His94, His96 and His119. The hydrophobic half of the active site is coloured in red, the hydrophilic one in blue. His64, the proton shuttle residue, in green. Active site ribbon view of hCA II in adduct with B) TPM and C) ZNS. H-bonds are represented as black dashed lines. Active site ribbon view of hCA II in adduct with B) topiramate and C) zonisamide. H-bonds are represented as black dashed lines.
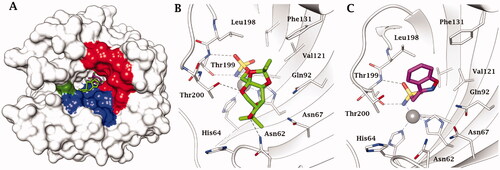
Table 2. Structures of compounds 24–33 screened for the inhibition of hCA I, II and VA.
Figure 6. hCA VA inhibitors 34–38 identified by VS techniques, and acipimox 39, identified by classical screening procedures.
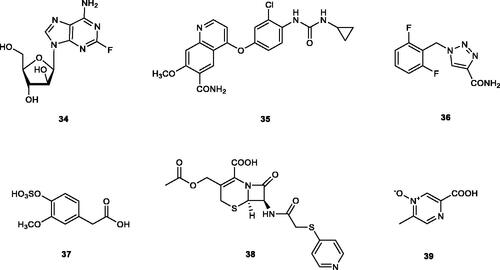
Table 3. Benzenesulfonamides and 1,3,4-thiadiazole-sulphonamides acting as low nanomolar hCA VA and VB inhibitorsCitation69.
Figure 8. Aromatic/heterocyclic sufonamides 49 and 50 incorporating 10-camphorsulfonyl tails. In derivatives 49, n = 0, 1 and 2. Triazole-sulphonamides 51 incorporate X, Y Z groups of the type H, F, Me, OMe, CF3, SO2NH2.
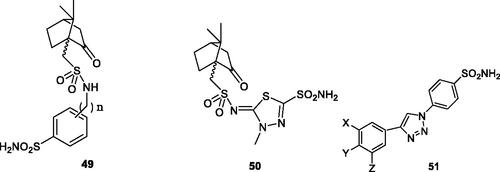
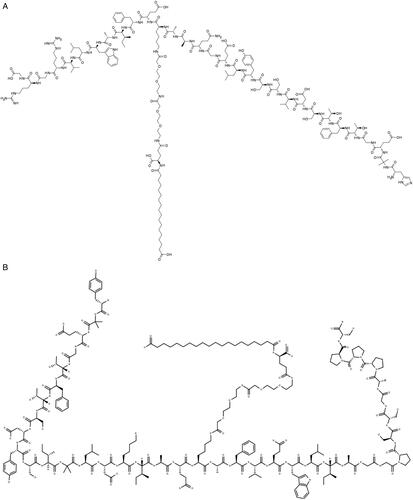
Figure 9. Chemical structures of Semaglutide (A), and Tirzepatide (B), recently approved anti-obesity agents.