Figures & data
Table 1. List of selected compounds including experimental techniques used to show direct interaction as well as observed effect(s) on VDAC1 and corresponding references.
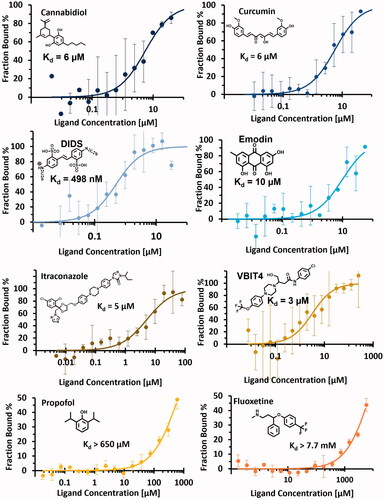
Table 2. Results obtained by NanoDSF and MST including Tm shift, MST binding check and dissociation constant evaluated for each tested compounds and published affinity if available. A thermal shift was considered significant when its variation exceeded ± 0.22 °C. (N.D., not determined, when experiments were not performed due to inconclusive binding check assay; N.A., not available, when experiments were performed but were inconclusive). hVDAC1 = human recombinant VDAC1 and rVDAC1 = VDAC1 extracted from rat.