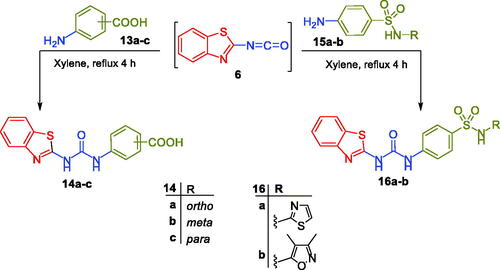
Figures & data
Table 1. Inhibition constants for benzothiazole-based derivatives (8a-c, 10, 12, 14a-c and 16a-b) and the standard sulphonamide inhibitor acetazolamide (AAZ) towards hCA I, II, IX and XII, determined with a stopped-flow CO2 hydrase assay.
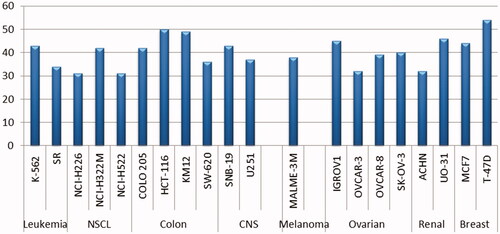
Table 2. Anticancer activity of target benzothiazole-based sulphonamide 8b towards breast T-47D and MCF-7 cancer cell lines under hypoxic conditions.