Figures & data
Table 1. Inhibition data (KI, nM) of human CA isoforms hCA I, II, IX and XII with compounds 4a–d, 5a–d, 6a–d, 12a–c, 13a–c, and 14a–c against SLC-0111 and AAZ; by a stopped flow CO2 hydrase assay.
Scheme 1. Reagents and reaction conditions: (i) SOCl2, methylene chloride, reflux, 4–5 h, (ii) NH4SCN, acetone, reflux, 1–3 h, (iii) sulphanilamide or 4-aminobenzoic acid or ethyl 4-aminobenzoate, acetone, reflux, 2–3 h.
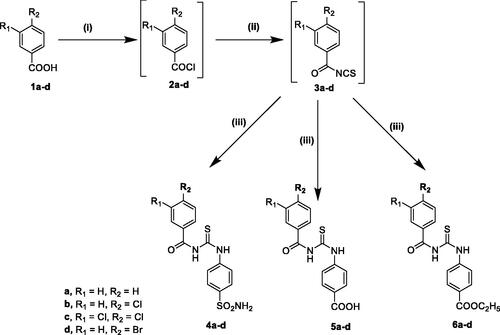
Scheme 2. Reagents and reaction conditions: (i) KOH, acetonitrile, R.T, 1–2 h, (ii), SOCl2, methylene chloride, reflux, 4–5 h, (iii) NH4SCN, acetone, reflux, 1–3 h, (iv) sulphanilamide or 4-aminobenzoic acid or ethyl 4-aminobenzoate, acetone, reflux, 2–3 h.