Figures & data
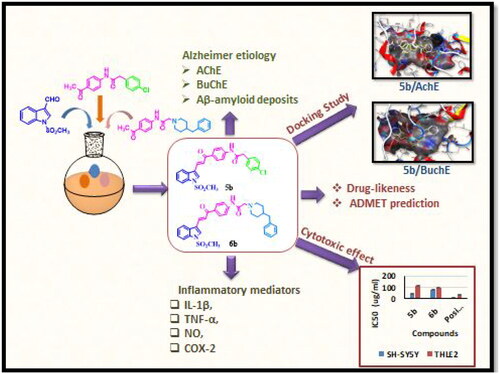
Figure 1. Examples of some drug candidates and natural products for the treatment of AD and anti-inflammatory agents and the design strategy for the novel derivatives.
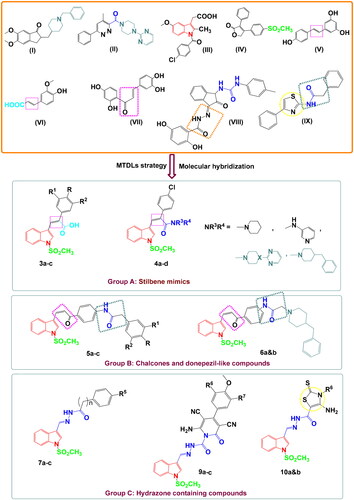
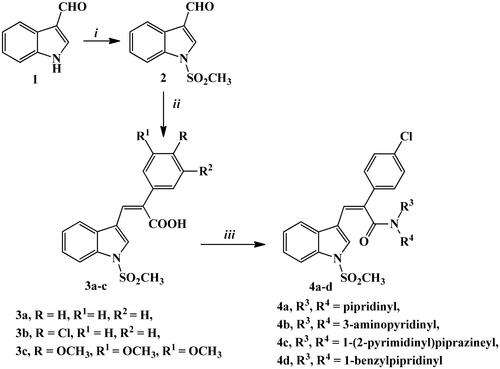
Scheme 1. Synthetic routes for preparation of starting material 2, carboxylic acid derivatives 3a–c, and amide derivatives 4a–d. Reagents and conditions: (i) NaH, ClSO3H, THF, stirring R.T., 3 h; (ii) phenyl acetic acid, p-chlorophenyl acetic acid or 3,4,5-trimethoxyphenyl acetic acid, K2CO3, Ac2O, 90 °C, 4–6 h; (iii) the appropriate amine, HBTU, DMF, stirring 2–4 h.
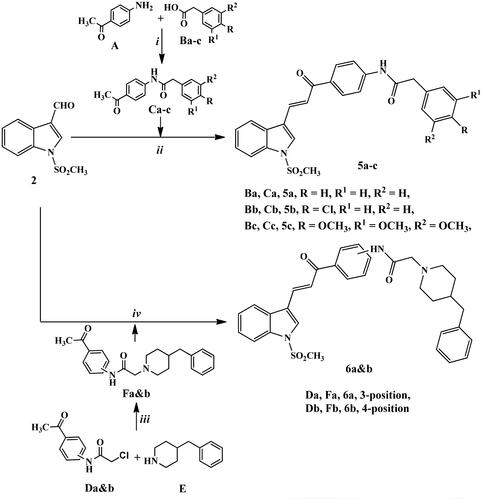
Scheme 2. Synthetic routes for preparation of chalcone derivatives 5a–c and 6a,b. Reagents and conditions: (i) HBTU, DMF, stirring 8 h; (ii) NaOEt, EtOH abs., stirring R.T., 24 h; (iii) K2CO3, KI, acetone, reflux, 6–8 h; iv) KOH, MeOH, stirring R.T., 24 h.
Scheme 3. Synthetic routes for preparation of imine derivatives 7a–c, 8, 9a–c, and 10a,b. Reagents and conditions: (i) benzohydrazide, phenylacetic acid hydrazide, or p-chlorophenyl acetic acid hydrazide, gl. acetic acid, reflux 3–5 h; (ii) cyanoacetic acid hydrazide, abs. ethanol, reflux 3 h; (iii) the appropriate arylidine derivative, abs. EtOH, reflux 4–6 h; (iv) ethyl/or phenyl isothiocyanate, S, abs. EtOH, TEA, reflux 10–12 h.
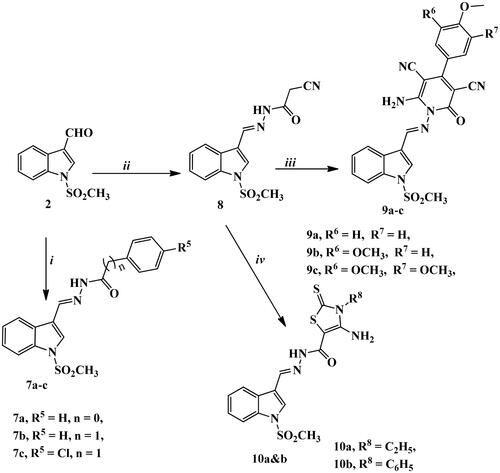
Figure 2. Acetylcholinesterase and butyrylcholinesterase (AChE and BuChE) inhibition activities for synthesised target compounds and reference drugs.
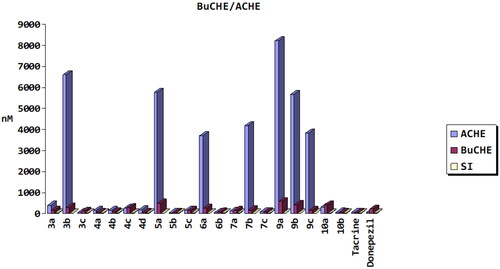
Table 1. Values of inhibition of Aβ1–42 self-induced aggregation for tested compounds and tacrine.
Figure 7. Cytotoxicity of synthetic compounds 5b and 6b and positive control on SH-SY5Y and THLE2 human cell lines.
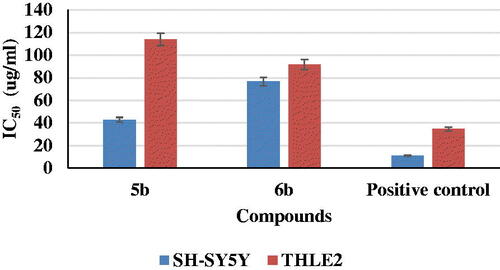
Figure 8. Binding interactions of the most active compound 5b inside rhAChE active site, (A) 3D image, 5b is described as white colour line and the ligand as yellow colour line, (B) 2D image.
Figure 9. Binding interactions of the most active compound 5b inside hBuChE active site, (A) 3D image, 5b is described as white colour line and ligand as yellow colour line, (B) 2D image.
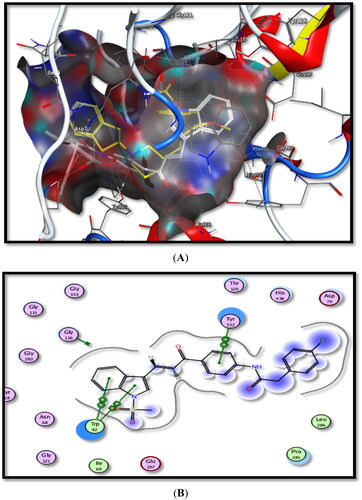
Table 2. Molecular modelling data for selected test compounds and ligands into rhAChE and hBuChE active sites.
Table 3. Predicted physicochemical properties and drug-likeness for some active compounds, donepezil and tacrine.
Table 4. In silico ADME prediction results for some active compounds, donepezil and tacrine.
Table 5. Predicted toxicity properties results for some active compounds, donepezil and tacrine.
Table 6. Metabolism prediction results for some active compounds, donepezil and tacrine.
Supplemental Material
Download PDF (1.4 MB)Data availability statement
The data supporting the findings of this study are available within the article [and/or] its supplementary materials.