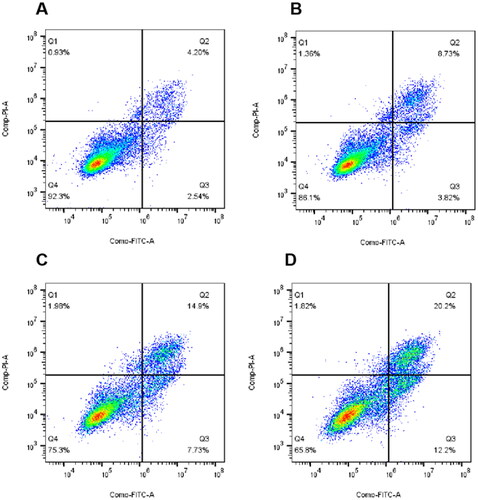
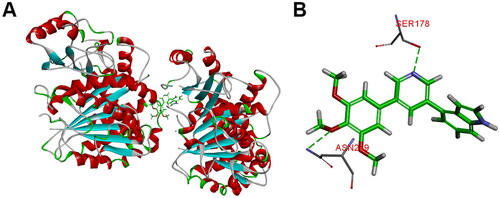
Figures & data
Scheme 1. Reagents and conditions (a) iPrMgCl.LiCl, iodine, piperidine, THF, –10 to 5 °C, 10 min; (b) 3,4,5-trimethoxybenzeneboronic acid, Pd(PPh3)4, K2CO3, 1,4-dioxane/H2O = 3/1, N2 atmosphere, 126 °C, M.W., 25 min; (c) substituted phenylboronic acid, Pd(PPh3)4, K2CO3, 1,4-dioxane/H2O = 3/1, N2 atmosphere, 126 °C, M.W., 25 min.
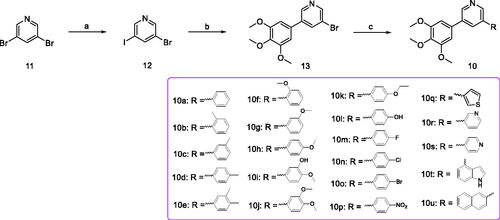
Table 1. Antiproliferative activity of all compounds.
Figure 3. Effect of 10t on tubulin polymerisation. Tubulin had been pre-incubated for 1 min with 10t at 10 and 15 μM, CA-4 at 5 μM, taxol at 5 μM or vehicle DMSO at room temperature before GTP was added to start the tubulin polymerisation reactions. The reaction was monitored at 37 °C.
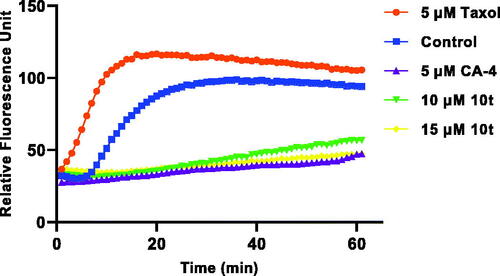
Figure 4. Effects of 2-fold IC50 CA-4 and 2-fold IC50 10t on the cellular microtubule networks of Hela cells by immunofluorescence assay. Microtubules and unassembled microtubule proteins stained with α-tubulin primary antibody and FITC secondary antibody, shown in green, and nuclei stained with DAPI, shown in the blue colour.
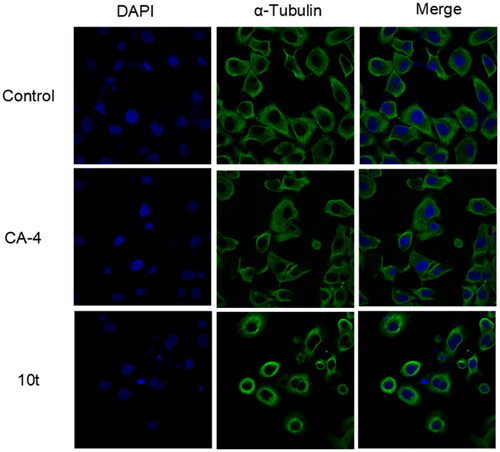
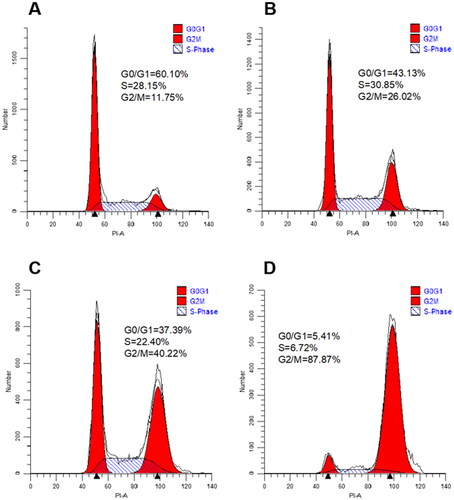
Figure 5. Cell cycle distribution of Hela cells after 24 h treatment with 1-fold IC50, 2-fold IC50 and 3-fold IC50 of 10t. (A) Control, (B) 1-fold IC50, (C) 2-fold IC50 and (D) 3-fold IC50.