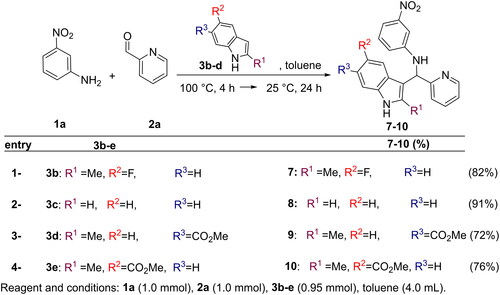
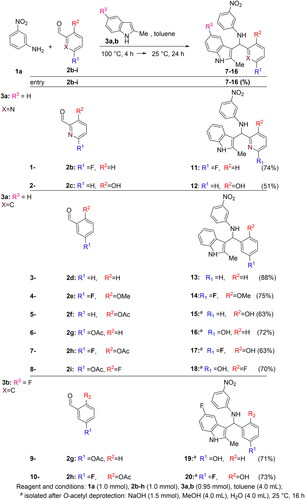
Figures & data
Figure 1. Chemical structure of the hit compound 4, and the binding modes predicted on EGFR by dockingCitation14.
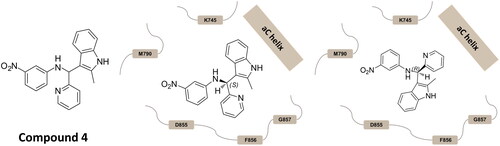
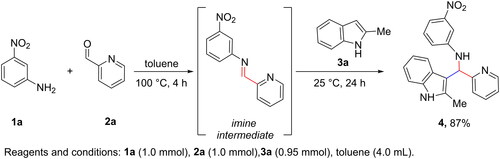
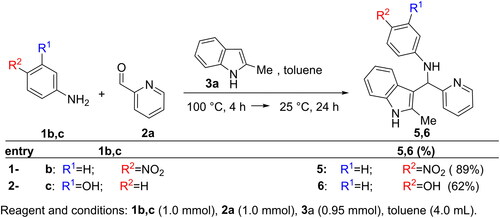
Table 1. Chemical structures of the synthesised compounds.
Figure 2. Activity of compounds in kinase assays. (A) Box and whiskers plot (min to max) of normalised IC50 values, relative to compound 4, from five independent experiments. (B) Dose-response curves of the indicated compounds from one representative experiment.
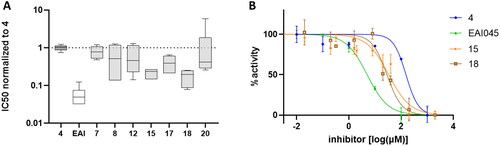
Figure 3. Activity of compounds 15 and 18 in H1975 cells. (A) Cell viability was measured by MTS assay. (B) EGFR phosphorylation was assessed by Western blot after 8-h incubation with the compounds (10 µM). Total EGFR and actin are shown for loading control.
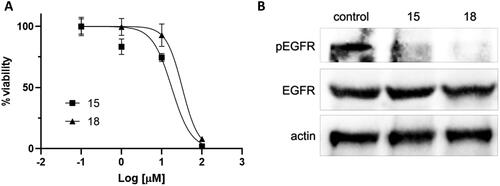
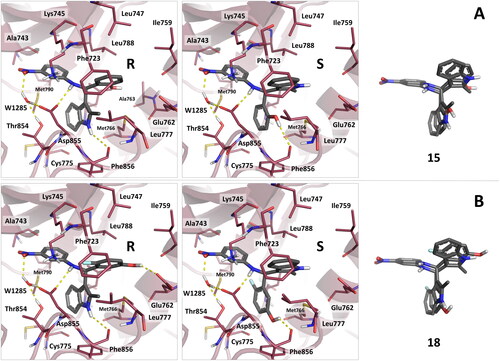
Figure 4. Binding mode predicted for compounds 15 and 18 by docking. (A) reports the binding mode predicted for the R and S stereoisomers of compound 15. (B) reports the binding mode predicted for the R and S stereoisomers of compound 18. The superimposition of the R and S stereoisomers of 15 and 18 in their predicted binding mode is also reported. As can be observed, the stereoisomers of the compounds show similar pattern of interactions, with good superimposition of their aromatic and h-bond donating groups.