Figures & data
Figure 1. The necessary pharmacophoric properties of some FDA and clinically approved VEGFR-2 inhibitors.
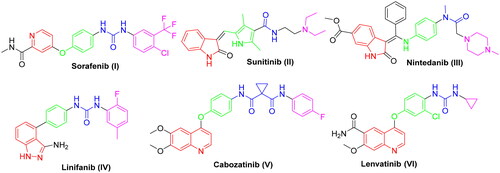
Figure 2. Representative examples of some reported anticancer VEGFR-2 inhibitors carrying 2-aminobenzothiazole scaffold, thiazolidine-2,4-dione, cyanothiouracil, and thiadiazole-urea pharmacophores.
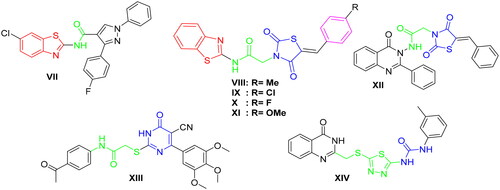
Figure 3. The fundamental structural requirements for SOR and rational of design of the novel postulated VEGFR-2 inhibitors.
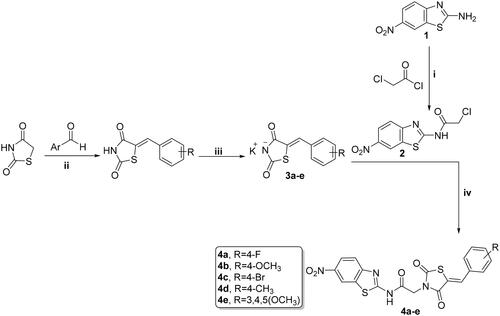
Scheme 1. Preparation of benzothiazole/thiazolidine-2,4-dione hybrids 4a–e; reagents and conditions: (i) CH2Cl2/Et3N 0 °C, rt; (ii) AcOH, NaOAc, reflux 14 h; (iii) EtOH, KOH, reflux 2 h; and (iv) DMF, K2CO3, reflux overnight.
Scheme 2. Synthesis of benzothiazole/1,3,4-thiadiazole-aryl urea hybrids 6a–d; reagents and conditions: (i) CS2, EtOH, reflux overnight; (ii) CH3CN, reflux overnight; and (iii) acetone, K2CO3, reflux overnight.
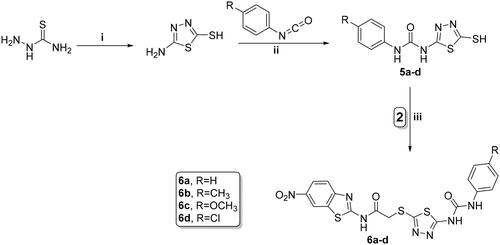
Scheme 3. Synthesis of benzothiazole/cyanothiouracil hybrids 8a–d; reagents and conditions: (i) EtOH, K2CO3, reflux 12 h and (ii) acetone, K2CO3, reflux overnight.
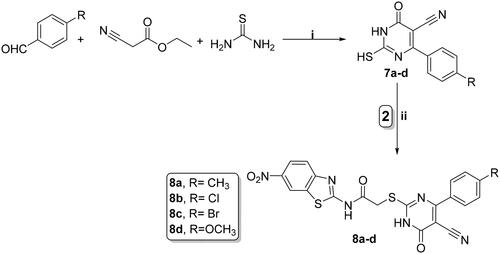
Table 1. Cytotoxicity (IC50) of the target hybrids 4a–e, 6a–d, and 8a–d towards HCT-116, HepG-2, MCF-7, and WI-38 cell lines.
Figure 4. Cytotoxic effect of the most active compounds 4a, 4e, 8e, and SOR on human normal WI-38 cell line.
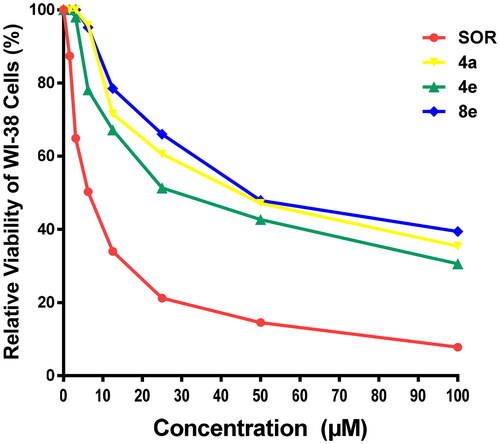
Table 2. Inhibitory effects of compounds 4a, 4e, and 8a against VEGFR-2.
Figure 5. Effect of compounds 4a, 4e, and 8a on DNA-ploidy flow cytometric analysis of MCF-7 cells. The cells were treated with DMSO as control and 4a, 4e, and 8a for 24 h.
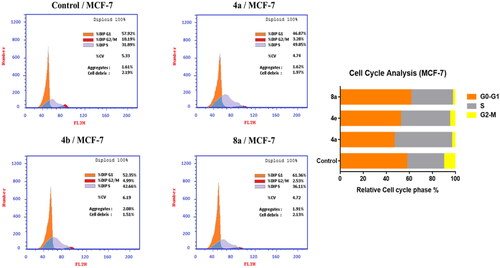
Table 3. Effect of compounds 4a, 4e, and 8a on the cell cycle progression in MCF-7 cells compared to SOR.
Figure 6. Effect of compounds 4a, 4e, and 8a on the percentage of annexin V-FITC-positive staining in MCF-7 cells. The cells were treated with DMSO as control and 4a, 4e, and 8a for 24 h. Q1 quadrant represents dead (necrotic) cells; Q2 quadrant represents late apoptosis; Q3 quadrant represent live cells; Q4 quadrant represents early apoptosis.
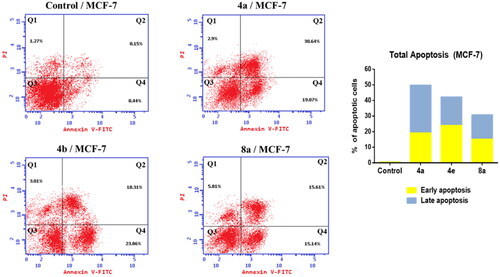
Figure 7. Compound 4a compound 4e compound 8a docked in the active site and aligned to the co-crystallized ligand SOR. Please refer to the online version for colored figure.
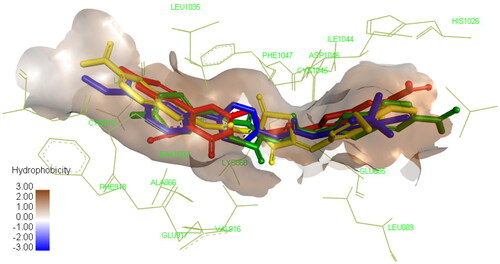
Table 4. Binding energy of the top three compounds docked in VEGFR-2 (PDB: 4ASD) in comparison to the co-crystallized ligand SOR.
Table 5. ADMET profile for active compounds 4a, 4e, 8a, and SOR.
