Figures & data
Figure 1. Discovery of 2-((4-sulfamoylphenyl)amino)-pyrrolo[2,3-d]pyrimidine derivatives as CDK inhibitors.
![Figure 1. Discovery of 2-((4-sulfamoylphenyl)amino)-pyrrolo[2,3-d]pyrimidine derivatives as CDK inhibitors.](/cms/asset/60a4466b-a052-40e1-9e01-267c973922d1/ienz_a_2169282_f0001_b.jpg)
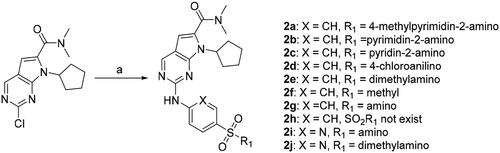
Scheme 1. Synthesis of 2-((4-sulfamoylphenyl)amino) substituted derivatives. Reagents and conditions: (a) arylamine, Xantphos, Pd2 (db)3, Cs2CO3, DMF, 110 °C, microwave irradiation.
Scheme 2. Synthesis of 2-((4-sulfamoylphenyl)amino) substituted derivatives. Reagents and conditions: (a) K2CO3, DCM,RT; (b) Pd/C, Methanol; (c) 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide, Xantphos, Pd2 (db)3, Cs2CO3, DMF, 110 °C, microwave irradiation; (d) 2 M hydrochloride in Methanol.
![Scheme 2. Synthesis of 2-((4-sulfamoylphenyl)amino) substituted derivatives. Reagents and conditions: (a) K2CO3, DCM,RT; (b) Pd/C, Methanol; (c) 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide, Xantphos, Pd2 (db)3, Cs2CO3, DMF, 110 °C, microwave irradiation; (d) 2 M hydrochloride in Methanol.](/cms/asset/3ad1e174-2db1-4565-86b0-fd9beefbfc85/ienz_a_2169282_sch0002_b.jpg)
Scheme 3. Preparation of 2-((4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)amino)-7H-pyrrolo[2,3-d]pyrimidine derivatives. Reagents and conditions: (a) 2-methoxyphenylamine, DIPEA, acetonitrile, 0 °C; (b) cyclopentylamine, acetonitrile;(c) 4-chloro-2-(methylthio)pyrimidine-5-carbaldehyde, DIPEA, acetonitrile, MW, 80 °C; (d) Cs2CO3, MW, 120 °C, 30 min, acetonitrile; (e) m-CPBA, DCM; (f) N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)formamide or N-(4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)formamide, Cs2CO3, DMSO, 85 °C
![Scheme 3. Preparation of 2-((4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)amino)-7H-pyrrolo[2,3-d]pyrimidine derivatives. Reagents and conditions: (a) 2-methoxyphenylamine, DIPEA, acetonitrile, 0 °C; (b) cyclopentylamine, acetonitrile;(c) 4-chloro-2-(methylthio)pyrimidine-5-carbaldehyde, DIPEA, acetonitrile, MW, 80 °C; (d) Cs2CO3, MW, 120 °C, 30 min, acetonitrile; (e) m-CPBA, DCM; (f) N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)formamide or N-(4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)formamide, Cs2CO3, DMSO, 85 °C](/cms/asset/7858819e-7d32-4dc3-bf39-c61a36aff643/ienz_a_2169282_sch0003_c.jpg)
Table 1. Anti-proliferative activity of 2-((4-sulfamoylphenyl)amino)-pyrrolo[2,3-d]pyrimidine derivatives in pancreatic cancer MIA PaCa-2 cell culture.
Table 2. Anti-proliferative activity of active 2-((4-sulfamoylphenyl)amino)-pyrrolo[2,3-d]pyrimidine derivatives in different PDAC cell lines.
Figure 2. Compound 2g induced slightly cell cycle arrest and late apoptosis of MIA PaCa-2 cells. The cells were incubated without or with 2g at indicated concentrations for 24 h. The cell cycle distribution (Upper lane) or apoptosis (Lower lane) was detected using propidium iodide (PI) staining or both PI and annexin V-FITC staining, respectively, and analysed by flow cytometry assays. Graphs showing the results of a representative experiment, and the data was presented as mean ± standard deviation.
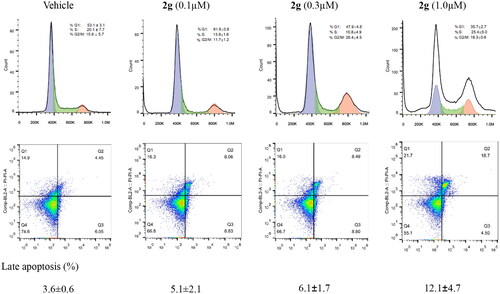
Figure 3. The anti-proliferative activity of new synthetic compounds is mainly mediated by CDK9. (A) 2g inhibited the phosphorylation of Rb and RNA polymerase II, induced apoptosis through down-regulation of Mcl-1 and c-Myc protein. MIA PaCa-2 cells were incubated with or without 2g at indicated concentration for 6 h. (B) CDK9 was dose-dependently knockdown by its specific siRNA after 48 h treatment in MIA PaCa-2 cells. (C) Downregulation of CDK9 using siCDK9 (50 nM) decreased the sensitivity of MIA PaCa-2 cell to compound 2g (0.2 µM). The cells were incubated with 2g for 72 h. NS no significance; ***p < 0.001; ****p < 0.0001. Whole-cell lysates were subjected to immunoblotting. A representative protein band of three independent experiments is shown. (D) Representative illustration of the binding of synthetic compound to CDK9/cyclin T. Small molecules 1 (left column), 2g (middle column), and 2i (right column) were shown in stick representation with carbons colored yellow.
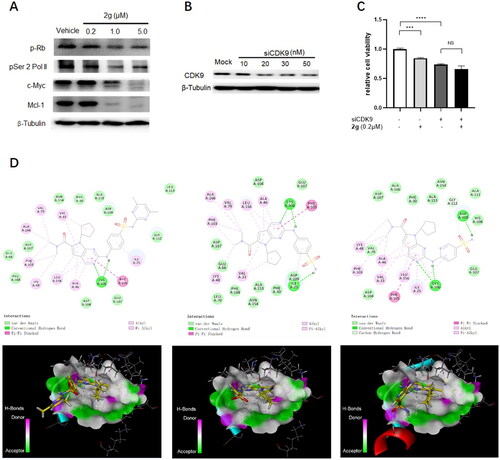
Table 3. IC50 (nM) values of anti-proliferative compounds against CDK activity.
Figure 4. Oral PK study of compounds 2g and 5 b after oral administration to rats (n = 3) at dose of 30 mg/kg.
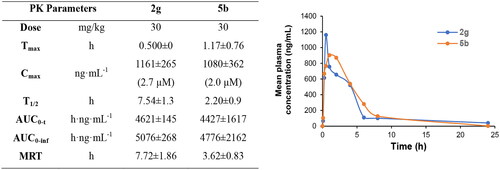
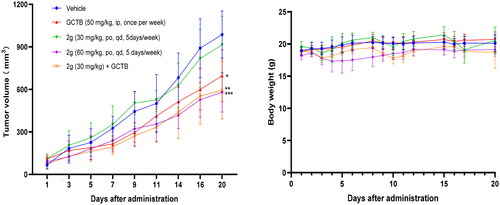
Figure 6. Representative H&E-stained tissue section (scale bar, 50 µM for all) from CDX mice treated with vehicle, GCTB or 2g alone, or combination of GCTB and 2g for 3 weeks.
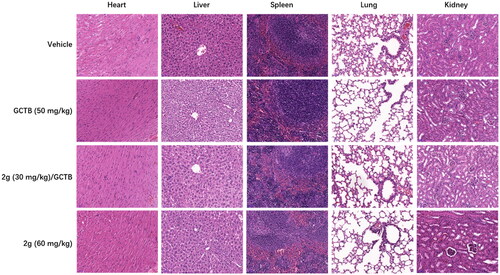
Table 4. Drug concentrations in tumour tissue and plasma sample after 30 mg/kg single dosing of 2g to tumour-bearing mice (n = 3).