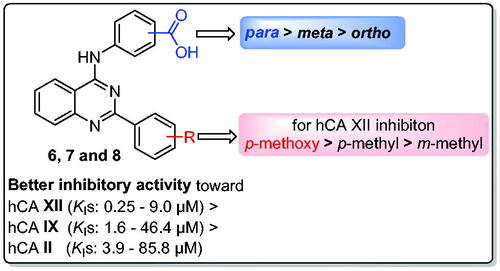
Figures & data
Figure 1. Structure of acetazolamide, dorzolamide, SLC-0111, non-classical CAIs (I–III), and the target inhibitors (6, 7, and 8).
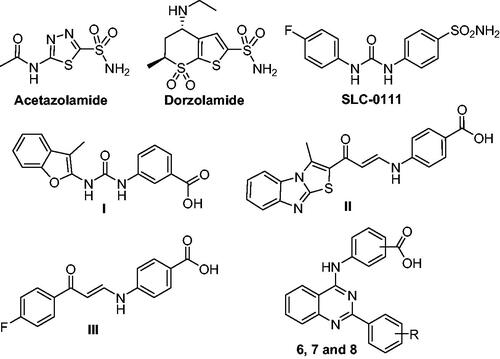
Scheme 1. Synthesis of chloroquinazolines (4a–c): Reaction conditions (i) FeCl3/H2O/heating 80 °C/3h, (ii) POCl3/N,N-dimethylformamide (cat.)/heating 90 °C/4h.
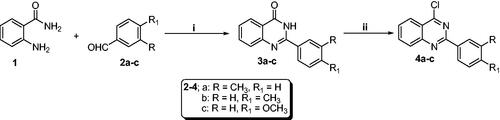
Scheme 2. Synthesis of 2-aryl-quinazolin-4-yl aminobenzoic acids (6a–c, 7a–c and 8a–c): Reaction conditions (i) Isopropanol/HCl (cat.)/reflux/2h.
Table 1. Inhibition data of hCA isoforms I, II, IX, XII, for carboxylic acids (6a–c, 7a–c, and 8a–c) by a stopped flow CO2 hydrase assay.
Table 2. Selectivity ratios for the inhibition of CA IX and XII isoforms over CA I and II isoforms for carboxylic acids (6a–c, 7a–c, and 8a–c) and acetazolamide.
Table 3. Percentage growth inhibition (GI%) of subpanel tumour cell lines at 10 μM concentration of the quinazoline based-carboxylic acids (6a–c, 7b, and 8a–b).
Table 4. GI50, TGI, and LC50 values of NCI five doses anticancer assay for 6b (NSC: 835857).
Table 5. Median GI50 values (µM) for compound 6b on subpanel tumour cell lines.