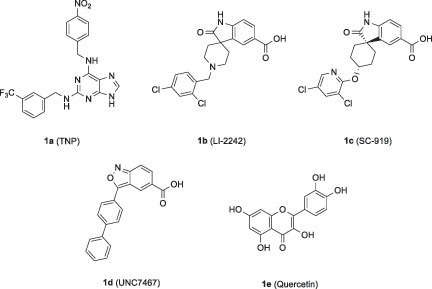
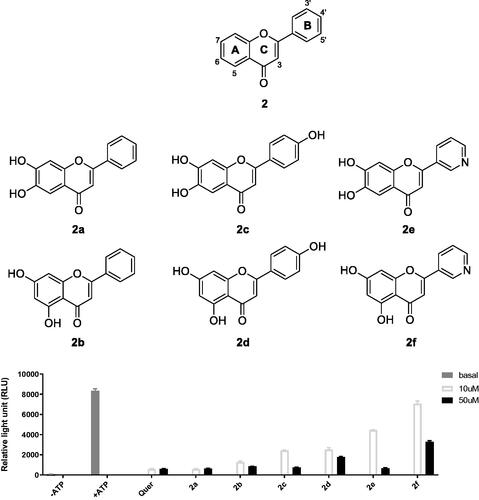
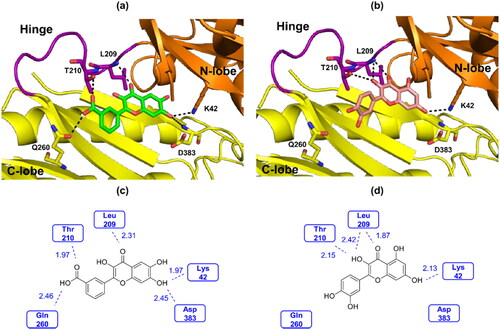
Figures & data
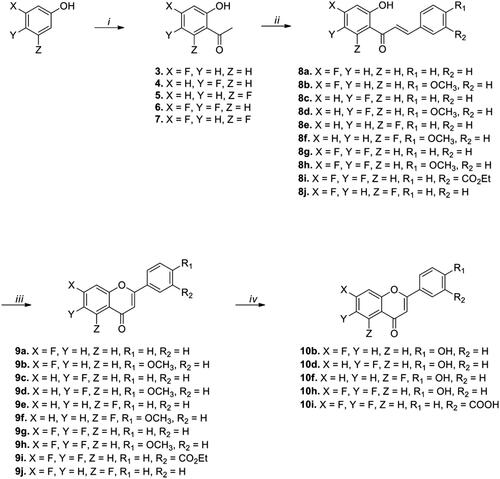
Scheme 1. Synthesis of fluoro-substituted flavonoid analogs with a variation of the B ring. Reagents and conditions: (i) (a) Acetyl chloride, pyridine, CH2Cl2, rt, 30 min, (b) AlCl3, 150 °C, 10 min; (ii) appropriate aldehydes, Ba(OH)2, MeOH (or EtOH), 50 °C, 1–17 h; (iii) I2, DMSO, 110 °C, 6–24 h; (iv) BBr3, CH2Cl2, 50 °C, 14–18 h.
Scheme 2. Synthesis of dihydroxy-substituted flavonoid analogs with a variation of the B ring. Reagents and conditions: (i) Appropriate aldehydes, Ba(OH)2, MeOH (or EtOH), 50 °C, 11–20 h; (ii) I2, DMSO, 110 °C, 11–17 h; (iii) BBr3, CH2Cl2, 50 °C, 5–18 h.
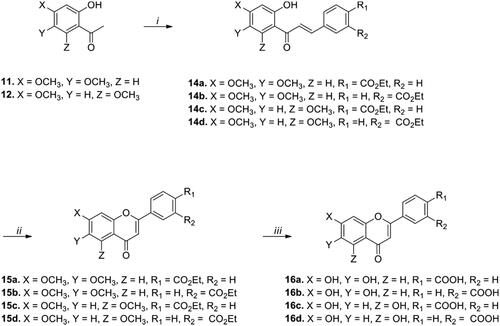
Table 1. IC50 values of the synthesised compounds 16a-16d against IP6K2.
Scheme 3. Synthesis of flavonoid analogs with a 3-OH group on the C ring. Reagents and conditions: (i) Appropriate aldehydes, NaOMe, THF, rt, 16–20 h; (ii) a. H2O2, NaOH (or NaOMe), EtOH (or MeOH), 40 °C, b. HCl, 16–20 h; (iii) BBr3, CH2Cl2, 50 °C, 6-16 h.
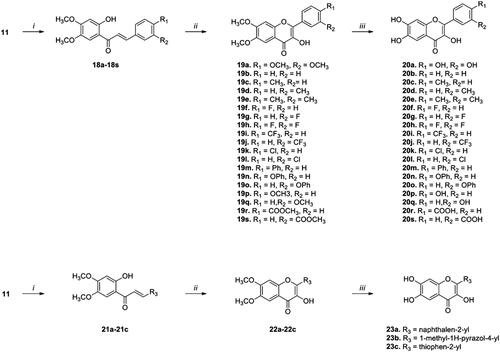
Table 2. IC50 values of the synthesised compounds against IP6K2.
Figure 3. (a) Dose-response curve of compound 20s against IP6K2. (b) Dose-response curve of quercetin against IP6K2.
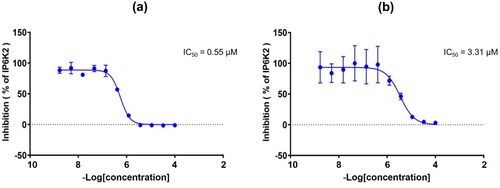
Table 3. Inhibition data of quercetin and compound 20s against IP6K1/2/3.