Figures & data
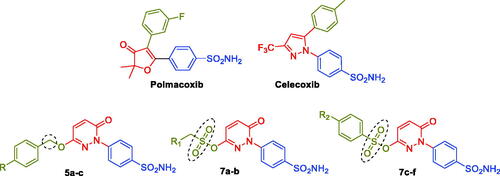
Figure 2. Chemical structures of the dual CA/COX-2 inhibitors Polmacoxib and Celecoxib, as well as the target pyridazinones 5a-c, and 7a-f.
Scheme 1. General Synthesis of pyridazine derivatives (2 and 5a-c); Reagents and conditions: (i) H2O, reflux, overnight; (ii) Potassium carbonate, DMF, stirring at 5 °C, 3 h.
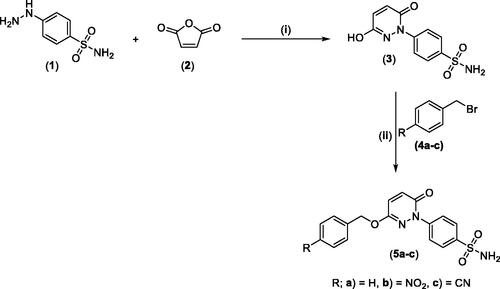
Scheme 2. General Synthesis of pyridazine derivatives (7a-f); Reagents and conditions: (i) Pyridine, stirring at 5 °C, 1 h.
Table 1. In vitro inhibition data of hCA I, II, IX and XII with pyridazine derivatives (3, 5a-c and 7a-f) by the stopped flow kinetic assay using AAZ as a reference drug.
Table 2. IC50 values for the in vitro COX-1/2 and LOX inhibition, as well as COX SI values of the pyridazine-based sulphonamide derivatives 3, 5a-c and 7a-f.
Table 3. Inhibition data of LOX enzyme with pyridazine-based sulphonamide derivatives 3, 5a-c and 7a-f.
Table 4. Analgesic impact of the tested methanesulfonate and ethanesulfonate pyridazines 7a and 7b by the use of acetic acid writhing test in mice.
Table 5. Results of the carrageen-induced paw edoema assay that was used to investigate the effects of the pyridazine sulphonates 7a and 7b on paw height.
Figure 3. Serum level of the inflammatory mediators (TNF-α and IL-1β) after paw edoema test. The value is expressed as mean ± SD (n = 5). *Significantly different from the positive control group. A) Tumour necrosis factor – alpha (TNF-α). B) Interleukin one beta (IL-1β).
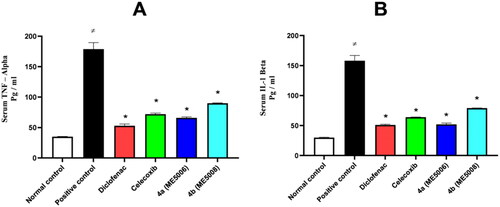
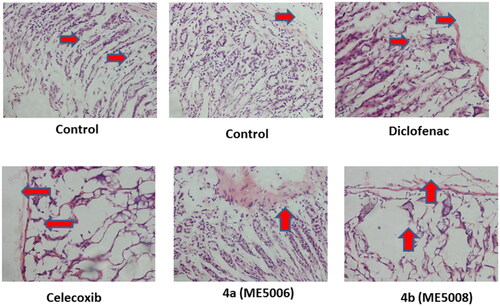
Figure 4. Ulcerogenic effects of the tested pyridazine sulphonates (7a and 7b), diclofenac and celecoxib on the gastric tissue of rats. Histopathological examination was performed using H & E stain and the magnification power of the images was 40X. The red arrows indicated the normal or damaged parts.