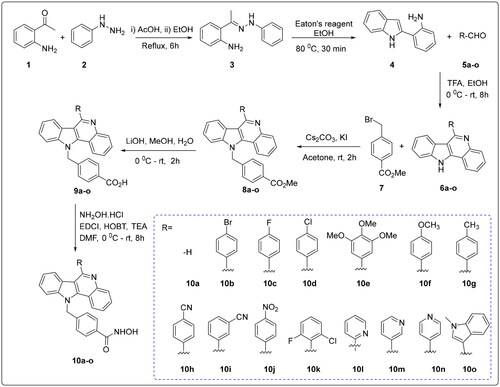
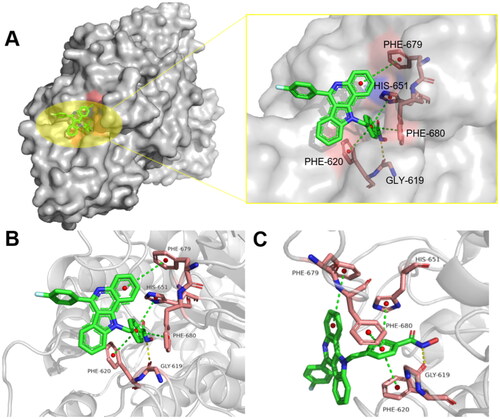
Figures & data
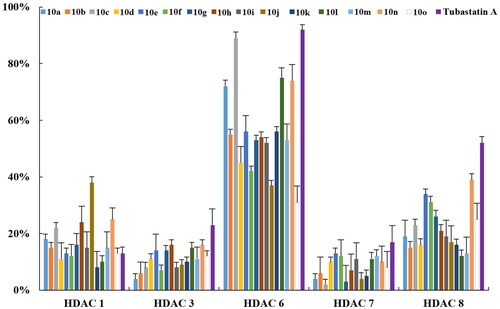
Table 1. The inhibitory activities of compounds against Hela nuclear extracts (at 1 μM).
Table 2. Overall inhibition of HDAC enzymes (IC50) in HeLa nuclear extracts by selected compounds with an inhibition rate of >50% at 1 μM.
Table 3. HDAC-inhibitory activity and isoform-selectivity of the newly synthesised compounds.
Table 4. In vitro antiproliferative activity of the newly synthesised compound.
Figure 5. 10c and 10l induced apoptosis in cancer cells. (A) Apoptosis of B16-F10 cells induced by increasing concentrations of 10c (2.5, 5.0, and 10.0 μM). (B) Histograms showing the percentages of cell cycle distribution following 10c treatment (n = 3). (C) Apoptosis of B16-F10 cells induced by increasing concentrations of 10l (2.5, 5.0, and 10.0 μM). (D) Histograms showing the percentages of cell cycle distribution following 10l treatment (n = 3). The bar graphs are presented as mean ± SD. ***p < 0.001, **p < 0.01, *p < 0.05 compared with the corresponding control group, One-way ANOVA analysis.
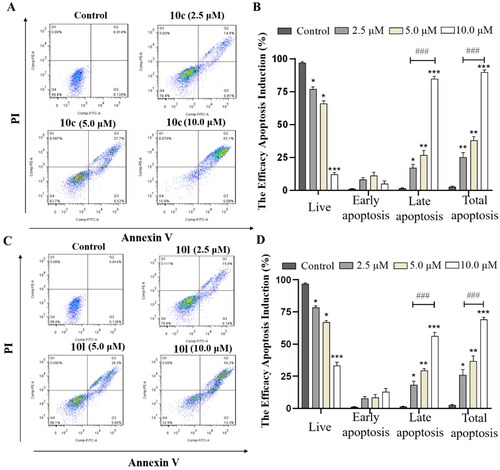
Figure 6. Induction of cell cycle arrest by 10c and 10l. (A) Cell cycle arrest induced by increasing concentrations of 10c (2.5, 5.0, and 10.0 μM). (B) Histograms showing the percentage of cell cycle distribution following 10c treatment (n = 3). (C) Cell cycle arrest induced by increasing concentrations of 10l (2.5, 5.0, and 10.0 μM). (D) Histograms showing the percentage of cell cycle distribution following 10l treatment (n = 3). The bar graphs are presented as mean ± SD. **p < 0.01, *p < 0.05 compared with the corresponding control group, one-way ANOVA analysis.
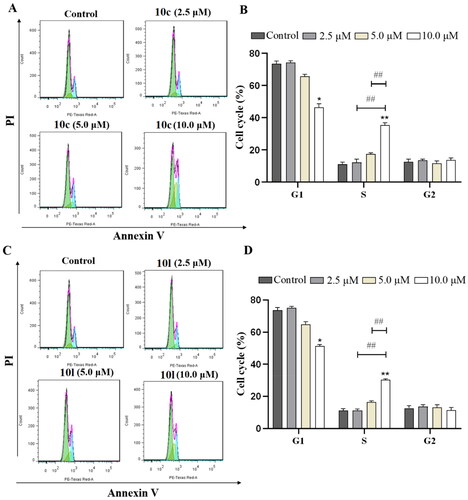
Figure 7. Analysis of Ac-α-tub and acetylated-H3 by western blot. The levels of Ac-α-tub and α-tubulin in B16-F10 cells (A) treated with DMSO or 10c, 10l, and tubastatin A for 6 h; quantitative analysis of the level of acetyl-α-tubulin (Ac-α-Tub) by western blotting assay obtained in B16-F10 cells (B). All data are representative of three independent experiments and shown as mean ± SD. ***p < 0.001, **p < 0.01 compared with the control group. One-way ANOVA for above analysis, Dunnett test.
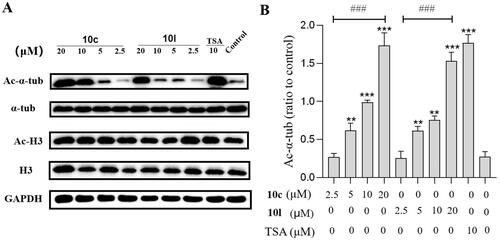
Figure 8. In vivo tumour-suppressing effects of 10c. Mice were treated with vehicle control, 10c (80 mg/kg), tubastatin A (80 mg/kg), NP19 (10 mg/kg), 10c + NP19 (40 mg/kg + 5 mg/kg per day) and tubastatin A + NP19 (40 mg/kg + 5 mg/kg per day) for 14 days consecutively, then sacrificed and tumours weights and volumes were measured. (A) The images of tumours; (B) weights of tumours. (C) changes of tumour volume during treatment; (D) changes of body weights of mice during treatment.
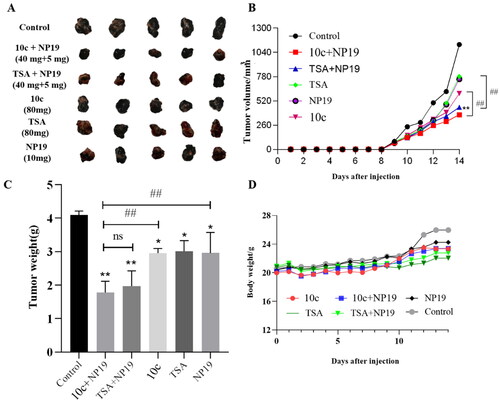
Figure 9. In vivo safety profiles of 10c. (A) Pathological analysis of tissue sections from major organs (heart, liver, and kidney) of melanoma tumour-bearing mice. Haematoxylin and eosin (H&E) were used to stain the organs with representative images captured; (B) blood cell analysis: white blood cell count (n = 6); (C) blood cell analysis: platelet count (n = 6).
Figure 10. Expression levels of Ac-α-tub protein in melanoma tumour tissues. Western blot analysis (A) and quantitative analysis (B) of Ac-α-Tub expression levels in melanoma tumour tissues collected from mice treated with vehicle control, 10c, NP19 + 10c, and NP19. n = 3, the bar graphs are presented as mean ± SD. One-way ANOVA for above analysis, Dunnett test.
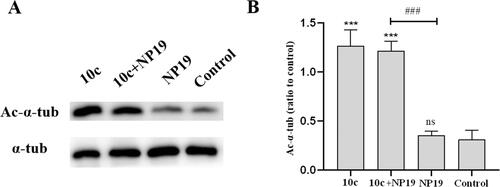
Figure 11. Expression levels of PD-L1 protein in melanoma tumour tissues. Western blot analysis (A) and quantitative analysis (B) of PD-L1 expression levels in melanoma tumour tissues collected from mice treated with vehicle control, 10c, NP19, and NP19 + 10c. n = 3, the bar graphs are presented as mean ± SD. One-way ANOVA for above analysis, Dunnett test.
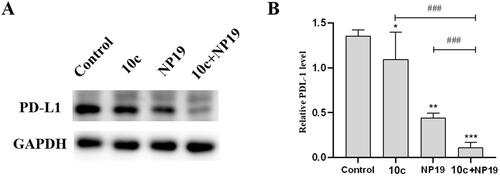
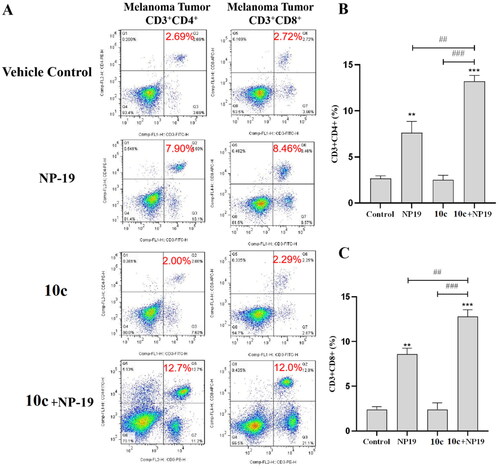
Figure 12. Detection of infiltrating lymphocytes in melanoma tumour tissues by flow cytometry. (A) Representative images for CD3+CD4+ cells (helper T cells) and CD3+CD8+ cells (activated cytotoxic T cells) in tumour tissues of vehicle control, 10c, NP19, (NP19 + 10c) combination-treated mice; melanoma tumour CD3+CD4+ (B) and CD3+CD8+ (C) cells in vehicle control, 10c, NP19, (NP19 + 10c) combination-treated mice. ***p < 0.001, **p < 0.01 compared with vehicle group (n = 6, Dunnett’s multiple comparison test).