Figures & data
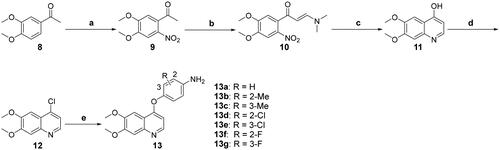
Scheme 1. Synthesis of the intermediates 13a–13g; reagents and conditions: (a) conc. HNO3 (40%), 0 °C, overnight; (b) DMF-DMA, toluene, reflux, 10 h; (c) Fe (powder), AcOH, 80 °C, 2 h; (d) POCl3, DMF (cat.), reflux, 1 h; (e) (1) 4-nitrophenols, PhCl, 140 °C, 20 h; (2) SnCl2, EtOH, 70 °C, 6 h.
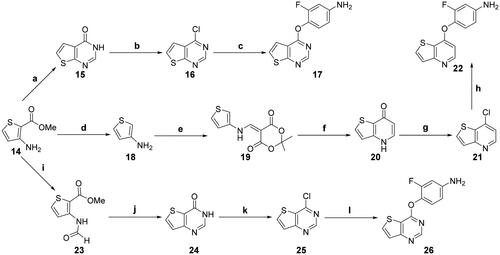
Scheme 2. Synthesis of the intermediates 17, 22, and 26; reagents and conditions: (a) formamide, 170 °C, 10 h; (b) POCl3, DMF (cat.), toluene, reflux, 6 h; (c) 4-amino-2-fluorophenol, NaH, DMF, 0 °C, 1.5 h; (d) (1) aq. NaOH, reflux, 30 min; (2) oxalic acid, 1-propanol, 38 °C, 45 min; (e) triethyl orthoformate, 2,2-dimethyl-1,3-dioxane-4,6-dione, 85 °C, overnight; (f) Ph2O, 240 °C, 30 min; (g) POCl3, DMF (cat.), 0 °C → reflux, 2 h; (h) (1) 2-fluoro-4-nitrophenol, K2CO3, Ph2O, 160 °C, 6 h; (2) SnCl2, MeOH, 70 °C, 6 h; (i) HCOOH, Ac2O, 0 °C → rt, 12 h; (j) HCONH2, 150 °C, 8 h; (k) oxalyl chloride, DMF (cat.), CH2Cl2, 0 °C → reflux, 3 h; (l) 4-amino-2-fluorophenol, NaH, DMF, 80 °C, 2 h.
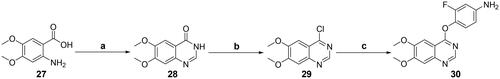
Scheme 3. Synthesis of the intermediate 30; reagents and conditions: (a) formamide, 150 °C, 8 h; (b) POCl3, 0 °C → reflux, 6 h; (c) 4-amino-2-fluorophenol, NaH, DMSO, 0 °C → 60 °C, overnight.
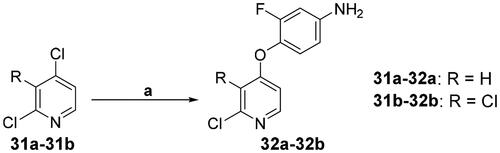
Scheme 4. Synthesis of the intermediate 32; reagents and conditions: (a) R = H, 4-amino-2-fluorophenol, NaH, DMF, 0 °C → 80 °C, overnight; R = Cl, 4-amino-2-fluorophenol, NaH, DMF, 0 °C → 100 °C, 3 h.
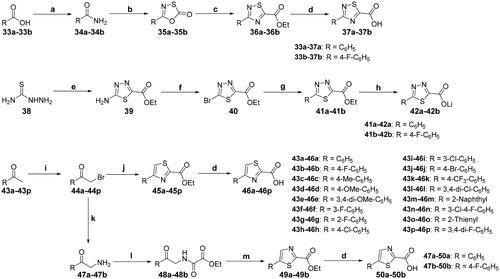
Scheme 5. Synthesis of the intermediates 37, 42, 46, and 50; reagents and conditions: (a) (1) SOCl2, 80 °C, 4 h; (2) NH4OH, THF, 0 °C → rt, 30 min; (b) (chlorothio)formyl chloride, toluene, 100 °C, 3 h; (c) ethyl cyanoformate, n-dodecane, 160 °C, 16 h; (d) aq. LiOH, MeOH, rt, 4 h; (e) ethyl oxalyl monochloride, POCl3, 70 °C, 6 h; (f) t-BuONO, CuBr2, CH3CN, 60 °C, 30 min; (g) Pd(OAc)2, Xantphos, NMM, toluene, H2O, rt, 7 h; (h) aq. LiOH, MeOH, 0 °C, 1 h; (i) NBS, p-TsOH, CH3CN, 50 °C, 24 h; (j) ethyl thiooxamate, EtOH, reflux, 6 h; (k) (1) urotropin, CHCl3, 50 °C, 2 h; (2) conc. HCl, EtOH, reflux, 2 h; (l) ethyl oxalyl monochloride, TEA, CH2Cl2, 0 °C → rt, overnight; (m) P2S5, CHCl3, reflux, 5 h.
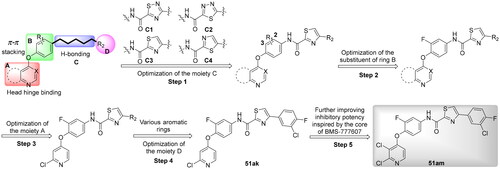
Scheme 6. Synthesis of target compounds 51a–51an; reagents and conditions: (a) oxalyl chloride, DMF (cat.), dry CH2Cl2, 0 °C → rt, 1.5 h; (b) corresponding amine, TEA, dry CH2Cl2, 0 °C → rt, 4–6 h; (c) corresponding amine, HATU, TEA, DMF, rt, overnight.
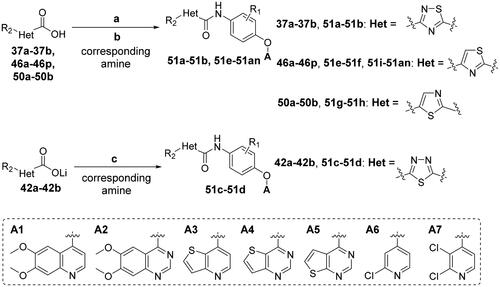
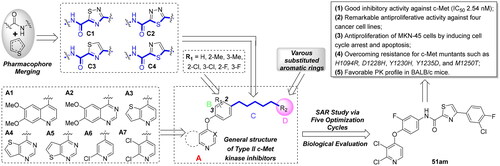
Table 1. In vitro c-Met inhibitory activities of target compounds 51a–51an.
Table 2. In vitro antiproliferative activities of compounds 51a–51an against six different human cell lines.
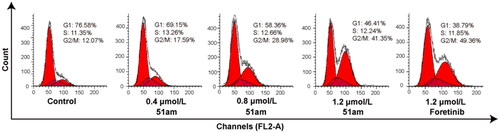
Figure 3. The effect of 51am and foretinib on MKN-45 cells apoptosis by Annexin V/PI double staining.
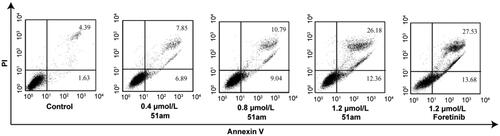
Table 3. c-Met mutant and kinase selectivity profile of 51am.
Table 4. Pharmacokinetic profile of 51am in BALB/c miceTable Footnotea.
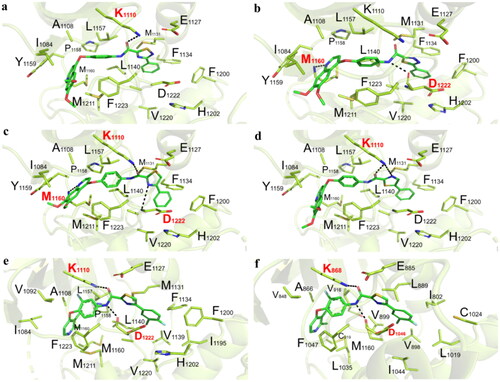
Figure 6. (a) The proposed binding mode of compound 51a (C1 scaffold as moiety C) with the active site of c-Met. Compound was shown in coloured sticks, green: carbon atom, blue: nitrogen atom, pink: oxygen atom, yellow: sulphur atom; (b) the proposed binding mode of compound 51c (C2 scaffold as moiety C) with the active site of c-Met; (c) the proposed binding mode of compound 51e (C3 scaffold as moiety C) with the active site of c-Met; (d) the proposed binding mode of compound 51g (C4 scaffold as moiety C) with the active site of c-Met; (e) the proposed binding mode of compound 51am with the active site of c-Met; (f) the proposed binding mode of compound 51am with the active site of VEGFR-2.