Figures & data
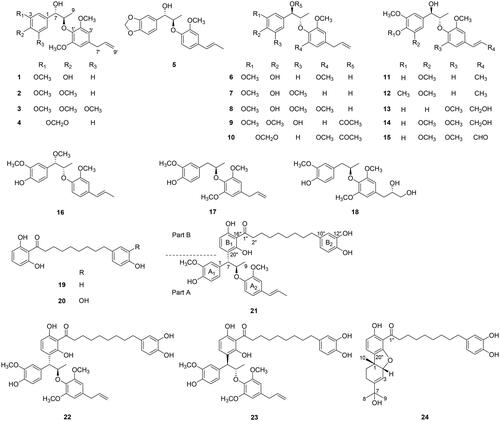
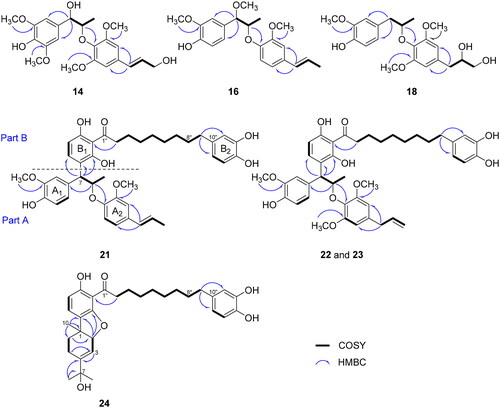
Table 1. NMR data (500 MHz, CDCl3) of compounds 14, 16, and 18.
Table 2. NMR data (500 MHz, CD3OD) of compounds 21–24.
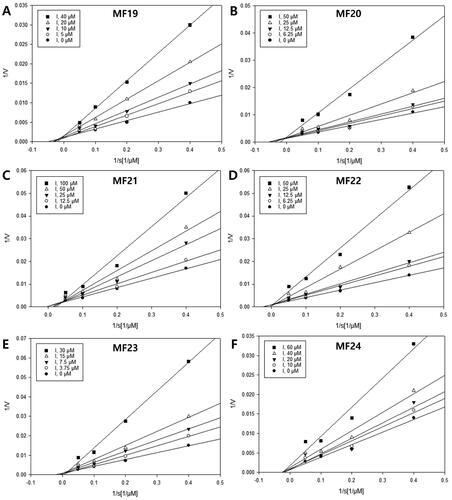
Table 3. sEH inhibitory activity, type of inhibitions, and inhibition constants of all isolated compounds (1–24).
Figure 4. (A–F) Docking interaction diagrams of sEH inhibition by compounds 19–24, respectively. (Green lines: hydrogen bonding interactions, pink lines: alkyl and π–alkyl interactions, magenta lines: π–π T-shaped interactions, and light green: van der Walls interactions with the corresponding residues of sEH).
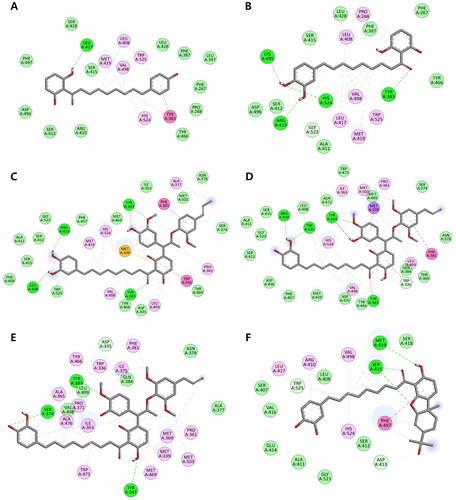
Figure 5. Molecular dynamics simulation of compounds 19–24 with sEH protein. (A) Potential energy, (B) RMSD of protein backbone (compounds 19: black, 20: red, 21: green, 22: blue, 23: yellow, and 24: maroon), (C–H) RMSF of residues in the complexes between sEH and compounds 19–24, respectively.
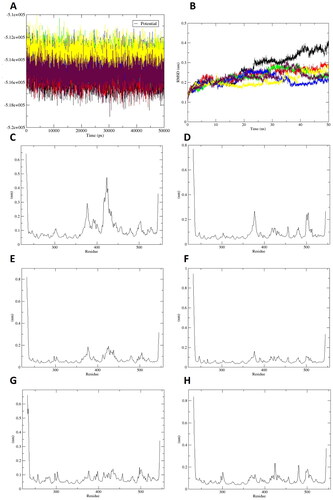
Figure 6. (A–F) Number of hydrogen bonds formed during molecular dynamics simulation by the complexes of sEH with compounds 19–24, respectively.
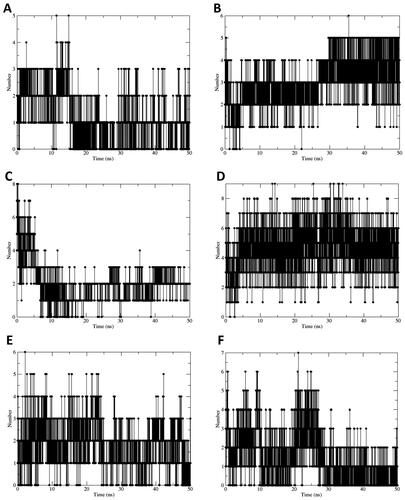
Table 4. Physicochemical and pharmacokinetic properties of compounds 19–24.