Figures & data
Figure 1. Structures of isatin, indole, and previously investigated isatin/indole-based AChEI and BChEI.
Table 1. Inhibition of AChE and BChE by N-alkyl isatins 4a-j and indoles 5a-j.
Figure 2. Lineweaver-Burk plot showing non-competitive inhibition of AChE with respect to acetylthiocholine (ATC) for compound 4i.
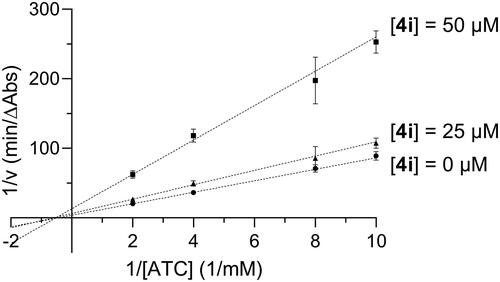
Figure 3. Lineweaver-Burk plot showing mixed inhibition of BChE with respect to butyrylthiocholine (BTC) for compound 4i.
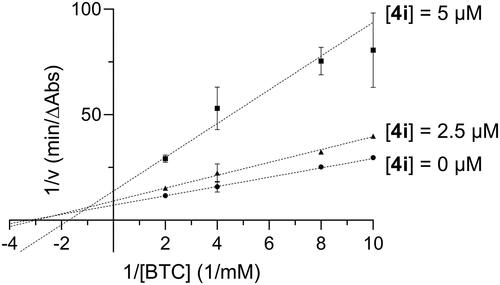
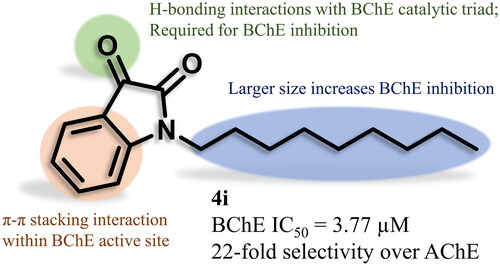
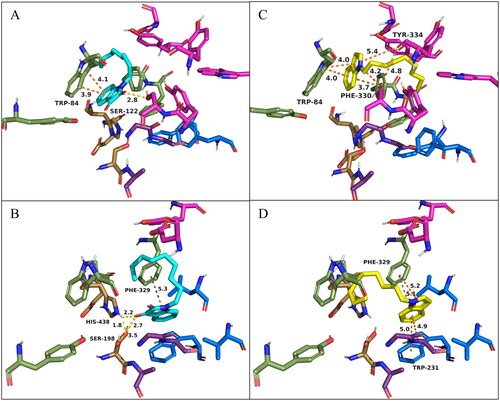
Figure 4. Docking of TcAChE (PDB: 1ACJ; A and C) and hBChE (PDB: 4BDS; B and D) with 1-nonylindoline-2,3-dione (4i, cyan) and 1-nonyl-1H-indole (5i, yellow). Active site residues of TcAChE include the catalytic triad (Ser200, His440, Glu327; gold), anionic site (Trp84, Tyr130, Phe330, and Phe331; green), oxyanion hole (Gly118, Gly119, Ala201; purple), acyl pocket (Phe288, Phe290; blue), and PAS (Tyr70, Asp72, Tyr121, Ser122, Trp279, Tyr334; magenta). Active site residues of hBChE include the catalytic triad (Ser198, His438, Glu325; gold), anionic site (Trp82, Tyr128, Phe329; green), oxyanion hole (Gly116, Gly117, Ala199; purple), acyl pocket (Trp231, Leu286, Val288; blue), and PAS (Asp70, Tyr332; magenta). Orange dashes indicate π-π stacking interactions, and yellow dashes indicate H-bonding interactions. All distances shown are in Å. O, N, and H atoms are shown in red, blue, and white, respectively.
Supplemental Material
Download PDF (2.2 MB)Data availability statement
The data that support the findings of this study are available from the corresponding author, TJE, upon reasonable request.