Figures & data
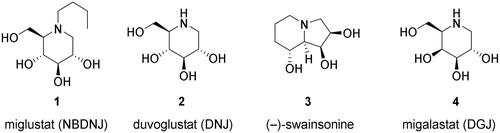
Figure 1. Selected glycosidase inhibitors that underwent clinical trials for various medical indications.
Scheme 1. Synthesis of deoxynojirimycin (DNJ)-derived α-glucosidase inhibitors. Reagents and conditions: a) R1CHO, H2 (1–4.2 atm), 10% Pd/C, 70–91%; b) H2 (1–4.2 atm), 10% Pd/C, 70–100%; c) n-BuBr, K2CO3, DMF, 80 °C, 69%; d) MeLi, –40 °C, Et2O, 100%; e) TBTH, AIBN, PhH, 80 °C, 88%; f) p-TsOH, MeOH, 95%; g) PPh3, I2, imidazole, THF, 88%; h) 74, K2CO3, DMF, 80 °C, 86%; i) H2 (4 atm), Pd(OH)2/C, HCl, MeOH, 98%. Ad = adamantane-1-yl.
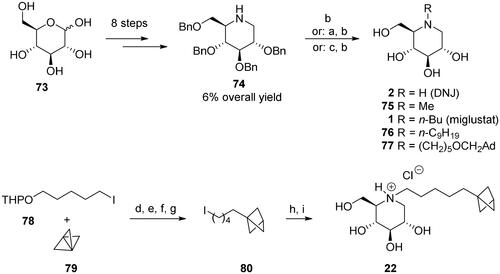
Figure 2. Structures and protonation states of the initial set of molecules at pH 7.00 ± 2.00, as predicted by the Epik module.
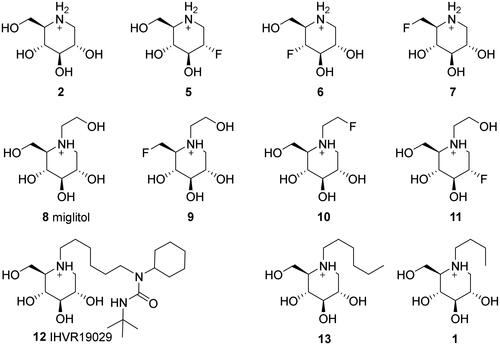
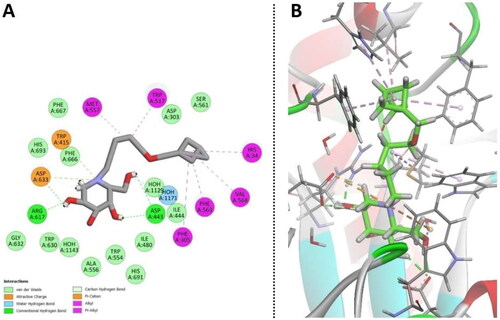
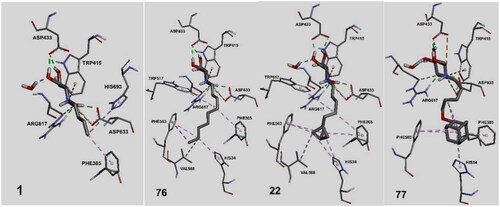
Figure 3. Binding interactions of small molecules with the binding site of endoplasmic reticulum (ER) α-glucosidase II (5DL0).
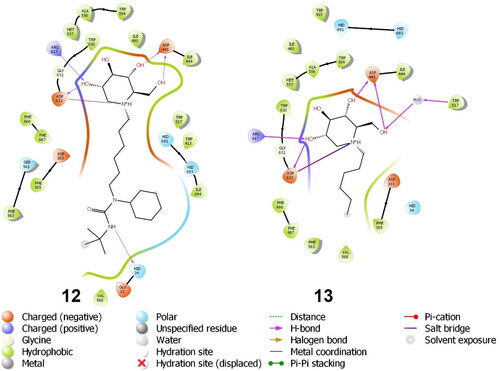
Table 1. Docking scores of the investigated compounds after docking (left) and refining (right) as well as the previously reported data on α-glucosidase inhibitors.
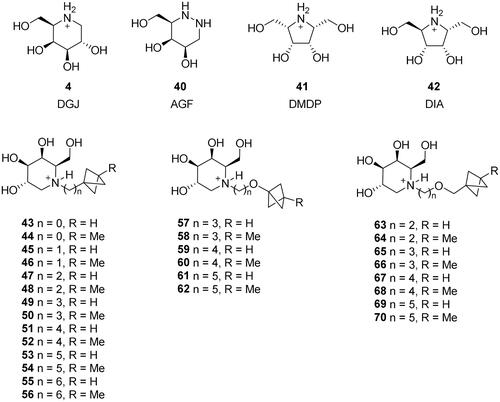
Figure 4. Structures and protonation states of the set of bicyclopentyl (BCP)-containing molecules at pH 7.00 ± 2.00, as predicted by the Epik module.
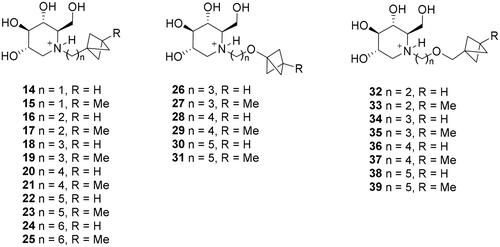
Scheme 2. Synthesis of deoxygalactonojirimycin (DGJ) and its analogues. Reagents and conditions: a) (R)-Pro (cat.), DMF, rt, 61%; b) H2 (4.5 atm), 10% Pd/C, EtOH, 75%; c) HCl, MeOH, 70–90%; d) RCHO, H2 (1–4 atm), 10% Pd/C, EtOH, 54–94%; e) (R)-Pro (cat.), DMF, 4 °C, 24 h, 60%; f) K2CO3, DMF, 80 °C, 95 : 96 = 2 : 1, 94% total yield; g) HCl, MeOH, 50%.
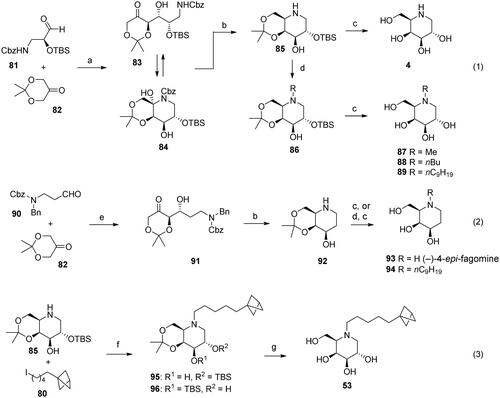
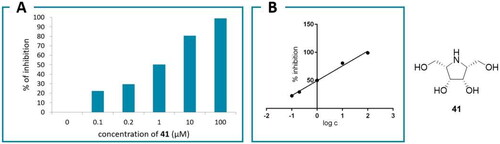
Scheme 3. Synthesis of pyrrolidine derivatives 41 and 42. Reagents and conditions: a) (R)-Pro (cat.), DMF, 4 °C, 48h, 98: 37%, 99: 33%; b) H2 (4.5 atm), 10% Pd/C, EtOH; c) 3 M HCl, MeOH, 42: 43% over 2 steps, 41: 55% over 2 steps.
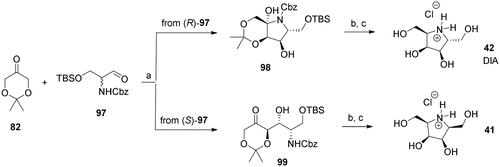
Scheme 4. Synthesis of azagalactofagomine (AGF) and its methylated derivative. Reagents and conditions: a) (S)-Pro, DMSO, H2O, 53%; b) H2 (1 atm), 10% Pd/C, MeOH, rt, 1 h; c) NaBH3CN, AcOH, MeOH, rt, 30 min, 65% over 2 steps; d) 3 M HCl(aq), MeOH, rt, 24 h, 40: 96%; 104: 100%; e) HCHO(aq), H2 (1 atm), Pd(OH)2/C, EtOAc, AcOH, rt, 6 h, 84%.
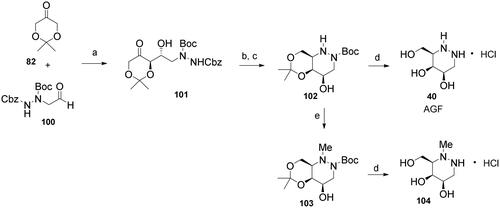
Scheme 5. Synthesis of (–)-swainsonine (3). Reagents and conditions: a) (R)-Pro (cat.), DMF, 66%; b) H2, Pd/C (10%), MeOH; c) CbzCl, Et3N, THF (71% over 2 steps).
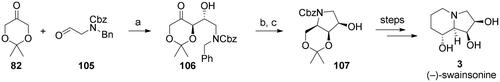
Table 2. IC50 values of the yeast α-glucosidase inhibitors.
Table 3. IC50 values of the human α-galactosidase inhibitors.
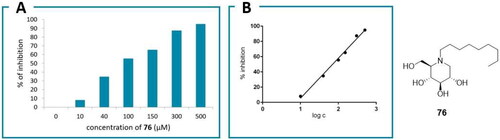
Figure 10. Anti-SARS-CoV-2 activities and cytotoxicities for α-glucosidase II inhibitors: 77 (A), 22 (B), 76 (C) and 1 (D).
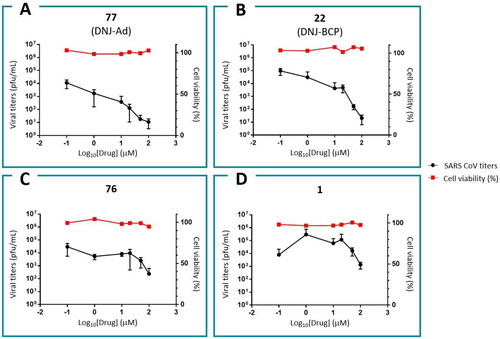
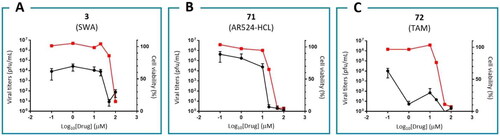
Figure 12. Antiviral activities and cell viabilities of mannosidase inhibitors: (−)-swainsonine (3, A), 71 (B), and tamoxifen (72, C).